A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
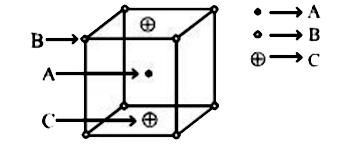
- In a solid A, B, C are arranged as above, the formula of solid is :
Text Solution
|
- In a crystallizes solid, anions B are arranged in cubic close packing,...
Text Solution
|
- A solide A^(+)B^(-) has the B^(-) ions arranged as below. If the A^(+)...
Text Solution
|
- In a crystalline solid, anions B arranged in ccp lattice and cations A...
Text Solution
|
- The interface betbeen a solid and gas may be represented by {:("(a) So...
Text Solution
|
- In a solid A, B, C are arranged as above, the formula of solid is :
Text Solution
|
- एक घनीय संरचना वाले ठोस के एकक सेल के कोनों पर A, केन्द्र पर B तथा फलक...
Text Solution
|
- एक ठोस जो कि A और B के मध्य बन्धों के द्वारा बनता है। इस ठोस में A तथा...
Text Solution
|
- A solid ABC has A, B and C arranged as below. The formula of solid is
Text Solution
|