Similar Questions
Explore conceptually related problems
Recommended Questions
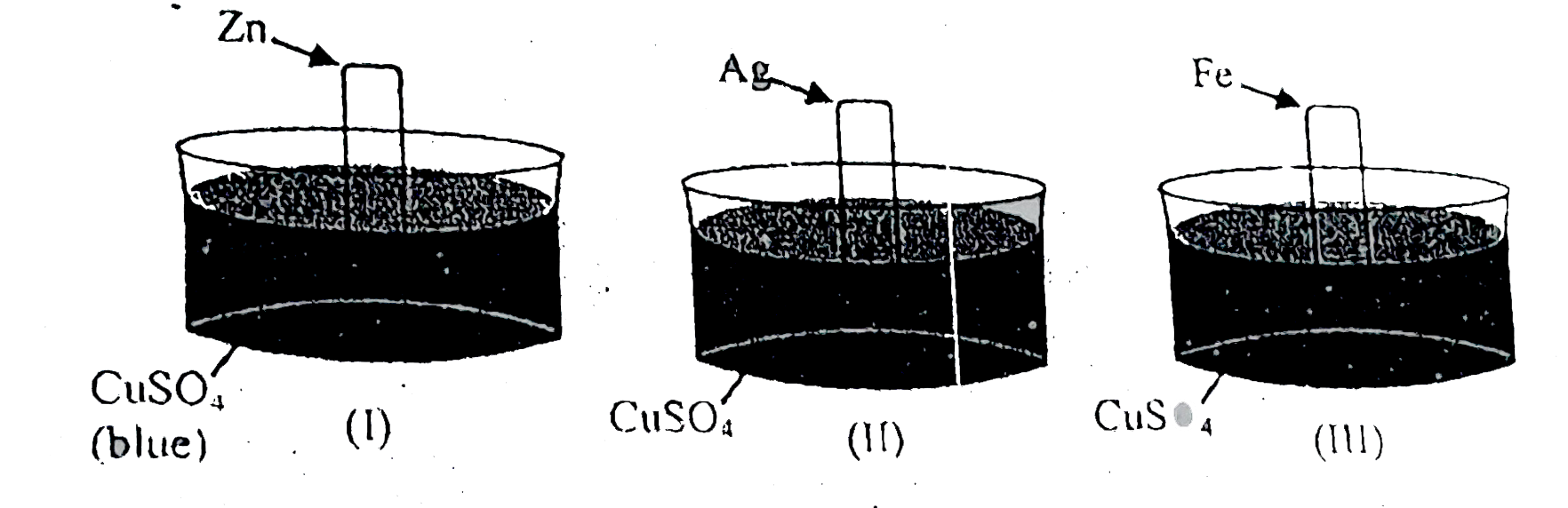
- Consider following sets : Blue colour solution changes to colourl...
Text Solution
|
- Consider following sets : Blue colour solution changes to colourless (...
Text Solution
|
- Which of the following solution has incorrect option of colour change,...
Text Solution
|
- "When NH(4)SCN (colourless) is added to dilute solution of FeCl(3) (li...
Text Solution
|
- If an iron rod is dipped into a solution of CuSO(4) , the blue colour ...
Text Solution
|
- The colour of the solution turns blue when a Cu - plate is dipped in a...
Text Solution
|
- Aqueous solution of CuSO4 is electrolysed using inert electrodes till ...
Text Solution
|
- Aqueous solution of CuSO4 is electrolysed using inert electrodes till ...
Text Solution
|
- Why blue colour of CuSO4 solution fades when an iron rod is dipped int...
Text Solution
|