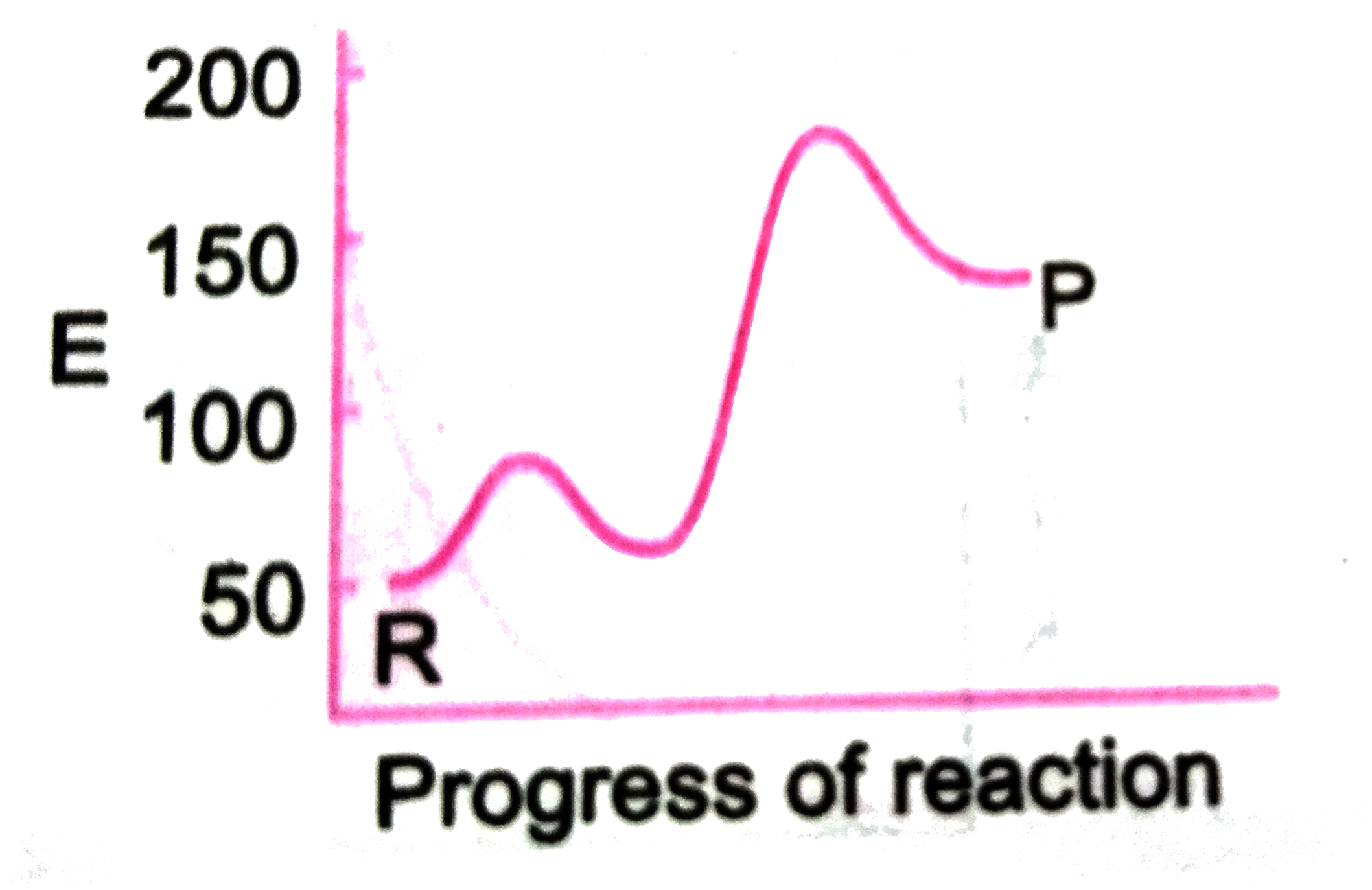
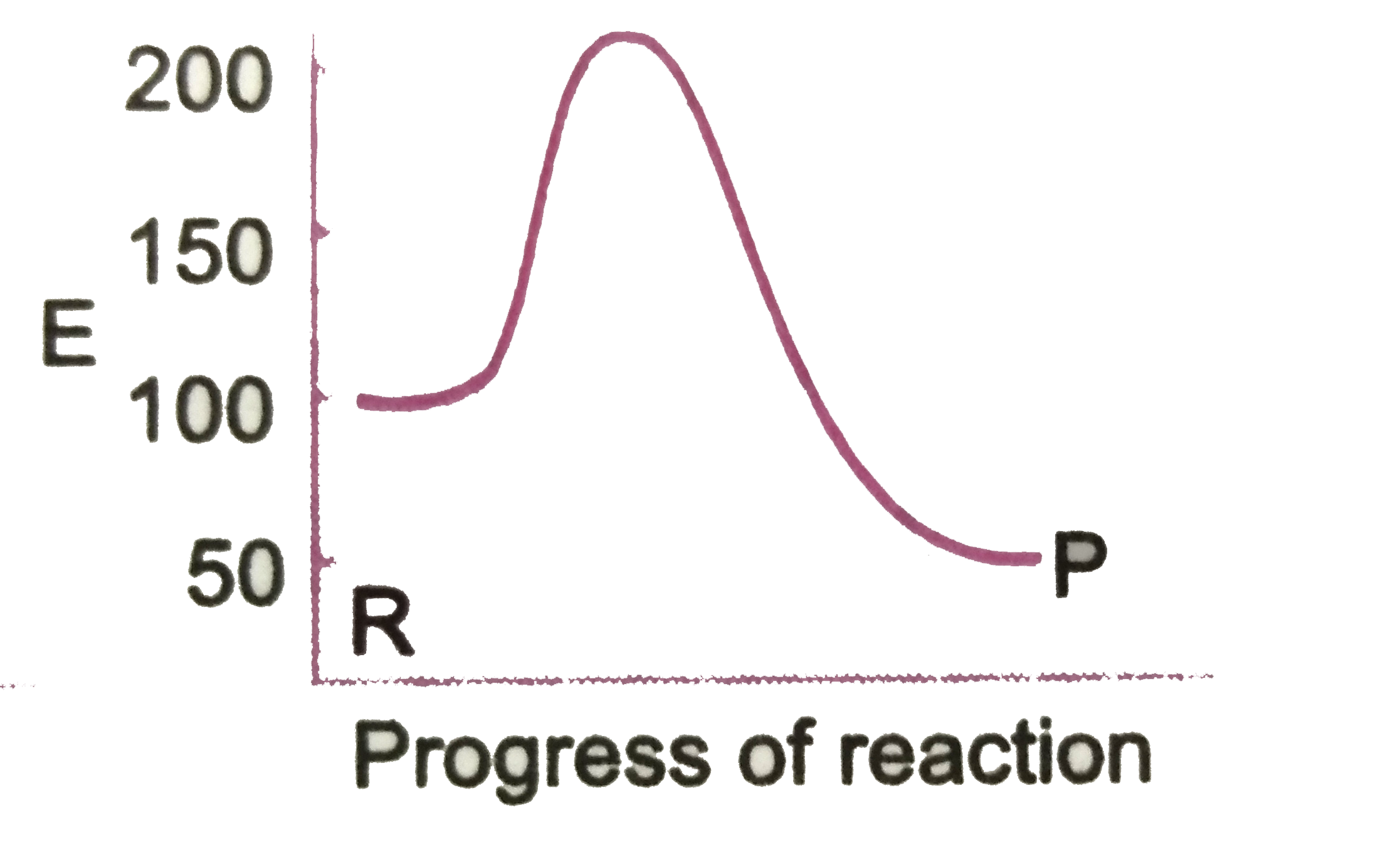
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL KINETICS
PRADEEP|Exercise COMPETITION FOCUS (JEE(Main and Advanced)/Medical Entrance) (II. Multiple Choice Questions)|1 VideosCHEMICAL KINETICS
PRADEEP|Exercise COMPETITION FOCUS (JEE(Main and Advanced)/Medical Entrance) (I. Multiple Choice Questions) With one or more than one correct answers|7 VideosCHEMICAL KINETICS
PRADEEP|Exercise VALUE BASED QUESTIONS (WITH ANSWERS)|2 VideosBIOMOLECULES
PRADEEP|Exercise IMPORTANT QUESTIONS (FOR BOARD EXAMINATION)|25 VideosCHEMISTRY IN EVERYDAY LIFE
PRADEEP|Exercise IMPORTANT QUESTION FOR BOARD EXAMINATION|30 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-CHEMICAL KINETICS-COMPETITION FOCUS (JEE(Main and Advanced)/Medical Entrance) (I. Multiple Choice Questions)
- The rate of a reaction doubles when its temperature changes form 300 K...
Text Solution
|
- The rate constant of a first orrder reaction becomes six times when th...
Text Solution
|
- A reactant (A) forms two products A overset (k(1))rarr B, Activation...
Text Solution
|
- In the presence of catalyst, the activation energy of the reaction is ...
Text Solution
|
- The rate constant of a reaction at temperature 200 K is 10 times less ...
Text Solution
|
- The formation of H(2)O(2) in the upper atmosphere follows the mechanis...
Text Solution
|
- For an endothermic reaction, where Delta H represents the enthalpy of ...
Text Solution
|
- When a catalyst increases the rate of a chemical reaction, the rate co...
Text Solution
|
- An exothermic chemical reaction proceeds by two stages reactants over...
Text Solution
|
- For a reaction taking place in three steps, the rate consatnt are k(1)...
Text Solution
|
- The rate constant k(1) and k(2) for two different reactions are 10^(16...
Text Solution
|
- For a first order reaction ArarrP , the temperature (T) dependent rate...
Text Solution
|
- Plots showing the variation of the rate constant (k) with temperature ...
Text Solution
|
- The activation energy for a reaction at temperature T K was found to b...
Text Solution
|
- Two reactions R(2) and R(2) have identical pre - exponential factors. ...
Text Solution
|
- Which one of the following is not correct ?
Text Solution
|
- One mole of N(2)O(4)(g) at 300 K is kept in a closed container under o...
Text Solution
|
- Consider the following statements. i) Increase in concentration of r...
Text Solution
|
- The oxidation of certain metal is found to obey the equation A^(2) =al...
Text Solution
|
- The decomposition of phosphine [PH(3)] on tungsten at low pressure is ...
Text Solution
|