Text Solution
Verified by Experts
Topper's Solved these Questions
SOME BASIC CONCEPTS OF CHEMISTRY
SUBHASH PUBLICATION|Exercise THREE MARKS QUESTION WITH ANSWERS|18 VideosSOME BASIC CONCEPTS OF CHEMISTRY
SUBHASH PUBLICATION|Exercise NUMERICAL PROBLEMS AND ANSWERS:|49 VideosSOME BASIC CONCEPTS OF CHEMISTRY
SUBHASH PUBLICATION|Exercise NUMERICAL PROBLEMS AND ANSWERS:|49 VideosS-Block Elements
SUBHASH PUBLICATION|Exercise Three marks questions and answers|8 VideosSTATES OF MATTER : GASES AND LIQUIDS
SUBHASH PUBLICATION|Exercise NUMERICAL PROBLEMS AND ANSWERS|25 Videos
Similar Questions
Explore conceptually related problems
SUBHASH PUBLICATION-SOME BASIC CONCEPTS OF CHEMISTRY -TWO MARKS QUESTIONS AND ANSWERS
- State Gay Lussac's law.
Text Solution
|
- Compute the mass of one molecule and the molecular mass of C(6)H(6) (b...
Text Solution
|
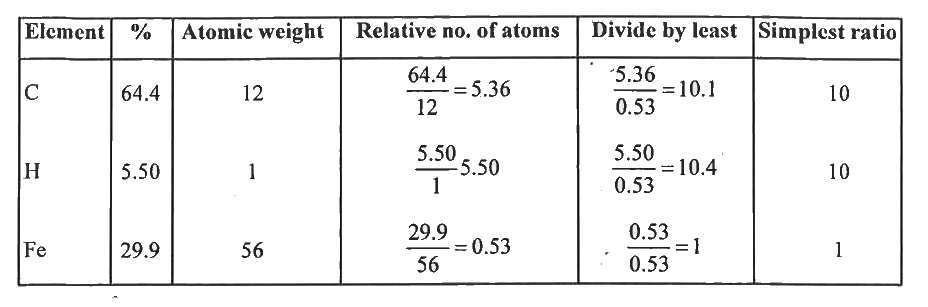
- An organometallic compound on analysis was found to contain, C = 64.4%...
Text Solution
|
- 4 g of copper chloride on analysis was founded to contain 1.890 g of c...
Text Solution
|
- Calculate the number of grams of oxygen in 0.10 mol of Na(2)CO(3).10H(...
Text Solution
|
- How many grams of Cl(2) are required to completely react with 0.4 g of...
Text Solution
|
- Conc. HCl is 38% HCl by mass. What is the molarity of this solution is...
Text Solution
|
- State Gay Lussac's law.
Text Solution
|
- Calculate the volume of O(2) at STP liberated by heating 12.25 g of KC...
Text Solution
|
- 1 M solution of NaNO(3) has density 1.25 g cm^(-3). Calculate its mola...
Text Solution
|
- Explain how compounds differ from elements? Give two differences.
Text Solution
|
- Explain how mixture differs from pure substances? Give 2 difference.
Text Solution
|
- Classify each of the following as pure substances or mixture, (a) Ethy...
Text Solution
|
- Define avogadro's law.
Text Solution
|
- Discuss average atomic mass with an example.
Text Solution
|
- Calculate the molecular mass of water (H(2)O) and water (O(2)) and oxy...
Text Solution
|
- Define Avogadro constant (N(A)).
Text Solution
|
- Define limiting reagent.
Text Solution
|
- What is mass percent (W/W%)
Text Solution
|
- What is mole fraction (X)
Text Solution
|