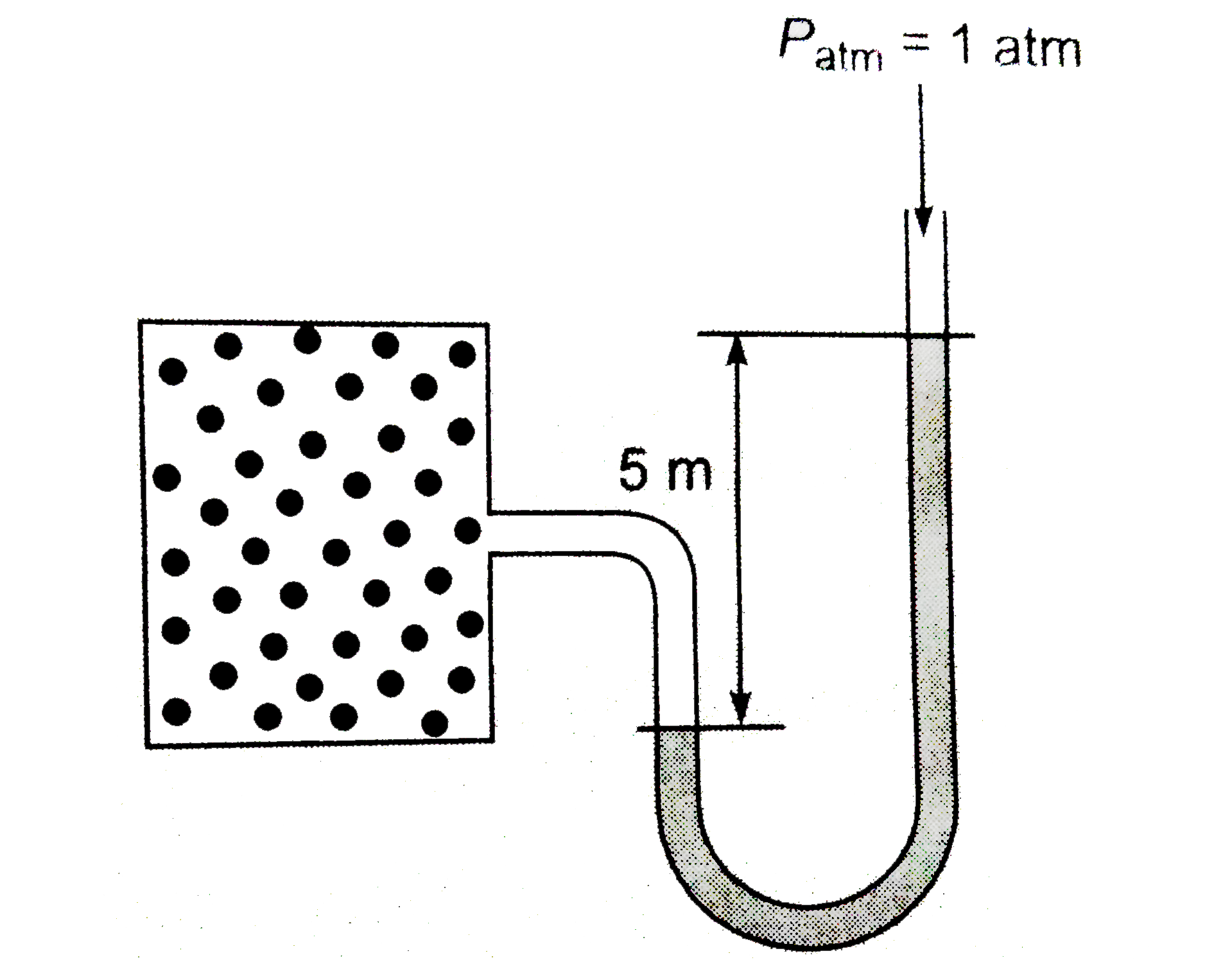Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MOTION-GASEOUS STATE -EXERCISE-3
- Automobile air bags are inflated with N(2) gas which is formed by the ...
Text Solution
|
- 3.6 gm of an ideal gas was injected into a bulb of internal volume of ...
Text Solution
|
- Calculate the number of moles of gas present in the container of volum...
Text Solution
|
- A toy balloon originally held 1.0 gm of He gas and had a radius 10 cm...
Text Solution
|
- While resting,the average human male use 0.2 dm^(3) of O(2)per hour at...
Text Solution
|
- An ideal gas is at a temperature of 200K& at a pressure of 8.21 atm.It...
Text Solution
|
- A manometer attached to a flask contains NH(3) gas have no difference ...
Text Solution
|
- 16 gm of O(2) was filled in a container of capacity 8.21 lit. at 300 ...
Text Solution
|
- (a) How much H(2)( in mol ) is needed to inflate a balloon of radius 3...
Text Solution
|
- Calculate the pressure of a barometer on an aeroplane which is at an a...
Text Solution
|
- What will be the temperature difference needed in a hot air balloon to...
Text Solution
|
- An iron cylinder contains helium at a pressure of 250 k pa and27^(@)C....
Text Solution
|
- Determine final pressure after the valve is left opened for along time...
Text Solution
|
- One mole of NH(4) Cl ( s) is kept in an open container & then covered ...
Text Solution
|
- Fixed mass of a gas is subjected to the changes as shown is diagram, c...
Text Solution
|
- A balloon containing 1 mole air at 1 atm initially is filled further w...
Text Solution
|
- One mole of an ideal gas is subjected to a process in which P =( 1)/( ...
Text Solution
|
- A compound exists in the gaseous phase both as monomer (A) and dimer (...
Text Solution
|
- During one of his adventure Chacha Chaudhary got trapped in an undergr...
Text Solution
|
- You are told to prepare a closed experimental environment ( a box) for...
Text Solution
|