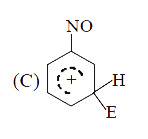
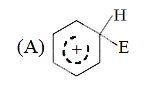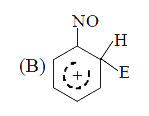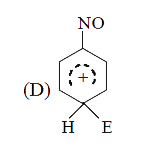A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MOTION-AROMATIC COMPOUND-Exercise - 4 | Level-I
- p-cresol reacts with chloroform in alkaline medium to give the compoun...
Text Solution
|
- The electrophile involved in the above reaction is :
Text Solution
|
- The structure of the compound that gives a tribromo derivative on trea...
Text Solution
|
- Fluorobenzene (C(6)H(5)F) can be synthesized in the laboratory ,
Text Solution
|
- Phenyl magnesium bromide reacts with methanol to give :-
Text Solution
|
- The reaction fo toluenec with Cl(2) in presence of FeCl(3) gives predi...
Text Solution
|
- The compound formed as a result of oxidation of ethyl benzene by KMnO...
Text Solution
|
- The electrophile, E^(o+) attacks the benzene ring to generate the inte...
Text Solution
|
- Toluene is nitrated and the resulting product is reduced with tin and ...
Text Solution
|
- Phenol when it first reacts with concentrated sulphuric acid and then ...
Text Solution
|
- Sodium phenoxide when heated with CO(2) under pressure at 125^(@) C y...
Text Solution
|
- In the following sequence of reactions: the product C is :
Text Solution
|
- Which of the following compounds will give significant amount of meta-...
Text Solution
|