Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
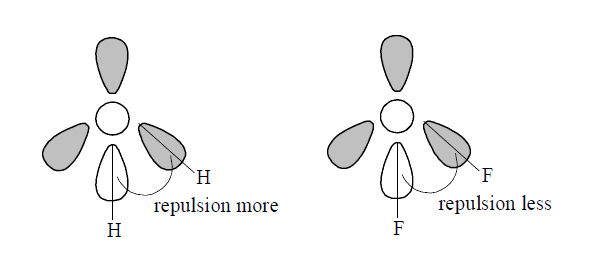
- Explain why the bond angle of H (2)O is 104 .5^(@) while that of F (2)...
Text Solution
|
- The O-O-H bond angle in H(2)O(2) is:
Text Solution
|
- Why is the H-O-H bond angle in H(2)O smaller than H-N-H bond angle in ...
Text Solution
|
- Why H-S-H bond angle in H(2)S is smaller than H-O-H bond angle in H(2)...
Text Solution
|
- Bond angles H-O-H and H-O-O- in water and H(2)O(2) respectively are
Text Solution
|
- The H - O - O bond angle in H(2)O(2) (g) is
Text Solution
|
- Explain why the bond angle of H (2)O is 104 .5^(@) while that of F (2)...
Text Solution
|
- Explain why the bond angle of H (2)O is 104 .5^(@) while that of F (2)...
Text Solution
|
- VSEPR theory suggests that bond length and bond angle depend upon the ...
Text Solution
|