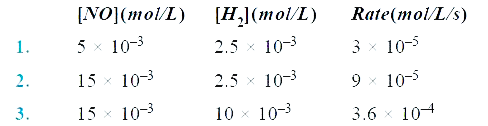A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- For a reaction 2NO(g) + 2H2(g) to N2(g) + 2H2O(g) , the following data...
Text Solution
|
- For a reaction 2NO(g) + 2H2(g) to N2(g) + 2H2O(g) , the following data...
Text Solution
|
- For a reaction 2NO(g) + 2H2(g) to N2(g) + 2H2O(g) , the following data...
Text Solution
|
- For a reaction 2NO(g) + 2H2(g) to N2(g) + 2H2O(g) , the following data...
Text Solution
|
- The following concentration were obtained for the formation of NH3 fro...
Text Solution
|
- At particular temperature KC = 4 xx 10^(-2) for the reaction H2S(g) hA...
Text Solution
|
- for the reaction 2H2(g)+2NO(g)rarrN2(g)+2H2O(g) the proposed mech...
Text Solution
|
- The following concentration were obtained for the formation of NH3 fro...
Text Solution
|
- For a reaction 2NO(g) + 2H2(g) to N2(g) + 2H2O(g) , the following data...
Text Solution
|