Similar Questions
Explore conceptually related problems
Recommended Questions
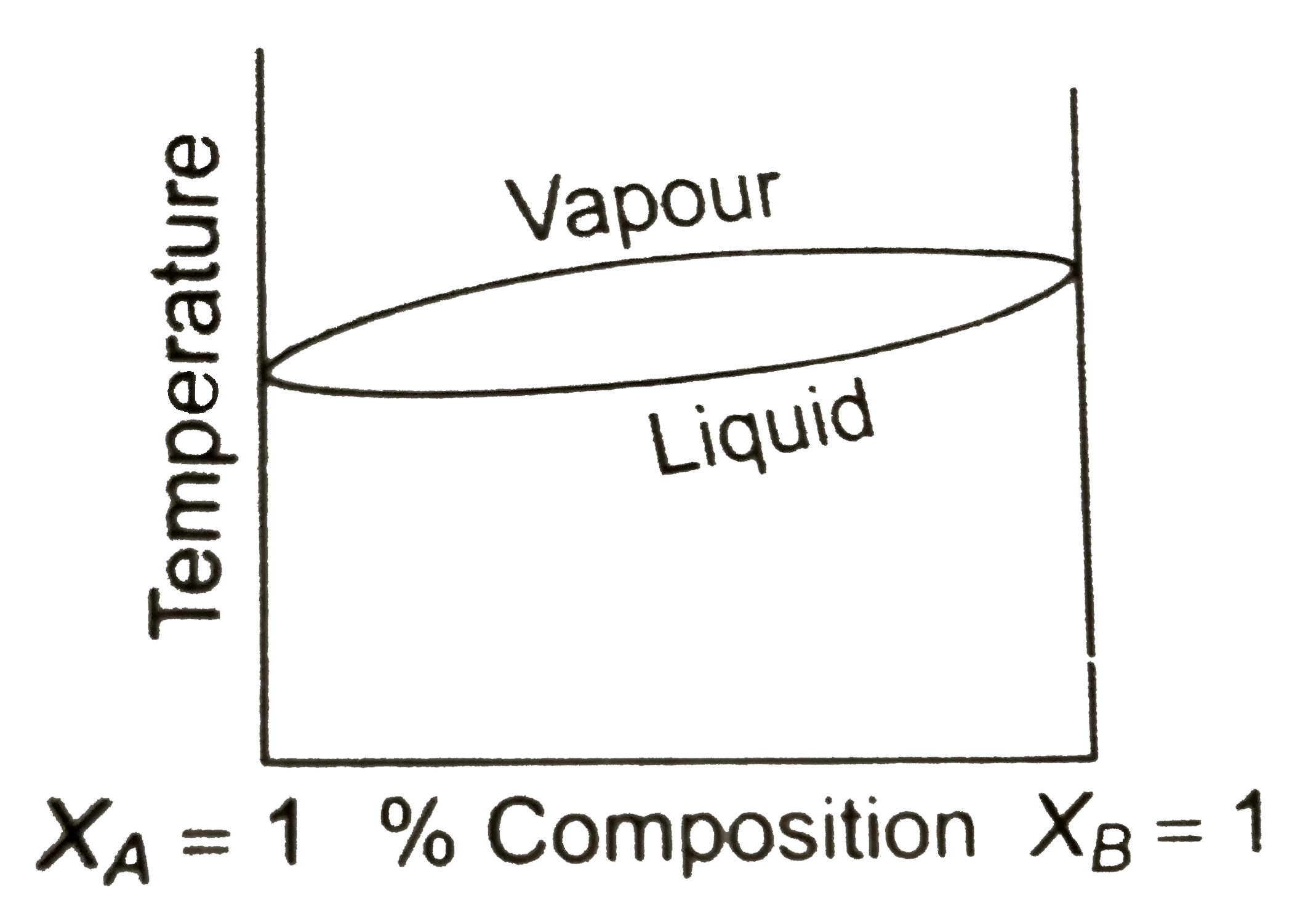
- Boiling point composition diagram of the liqid-vapour equilibrium for ...
Text Solution
|
- Azoetropes are constant boiling mixtures, which like a pure chemical c...
Text Solution
|
- The vapour pressure of pure liquid A at 300K is 577 Torr and that of p...
Text Solution
|
- Boiling point composition diagram of the liqid-vapour equilibrium for ...
Text Solution
|
- A liquid mixture ohaving composition corresponding to point Z in the f...
Text Solution
|
- Two liquids (A) and (B) can be separated by the method of fractional d...
Text Solution
|
- Which of the following binary mixture will have same composition in li...
Text Solution
|
- Two liquids (A) and (B) can be separated by the method of fractional d...
Text Solution
|
- Which of the following binary mixture will have same composition in li...
Text Solution
|