A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMODYNAMICS
MODERN PUBLICATION|Exercise Multiple Choice Questions (Level-II)|85 VideosTHERMODYNAMICS
MODERN PUBLICATION|Exercise Multiple Choice Questions (Level-III)|10 VideosTHE SOLID STATE
MODERN PUBLICATION|Exercise RECENT EXAMINATION QUESTIONS|11 VideosUNIT TEST - 3
MODERN PUBLICATION|Exercise MULTIPLE CHOICE QUESTIONS|59 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-THERMODYNAMICS-Recent Examination Question
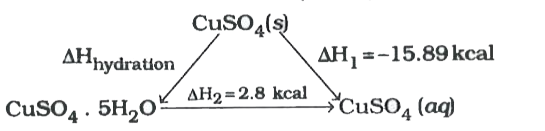
- The enthalpies of solution of anhydrous CuSO(4) and CuSO(4). 5 H(2)O a...
Text Solution
|
- Enthalpy of vaporization of benzene is + 35.3 kJ mol^(-1) at its boili...
Text Solution
|
- For the reversible reaction : A(s) + B(g) hArr C(g) + D(g) : Delta ...
Text Solution
|
- The amount of heat evolved when 500 cm^(3) of 0.1 M HCl is mixed with ...
Text Solution
|
- During the adsorption of krypton on activated charcoal at low temperat...
Text Solution
|
- Based on the first law of thermodynamics, which one of the following i...
Text Solution
|
- A gas expands from a volume of 1 m^(3) to a volume of 2m^(3) against a...
Text Solution
|
- Which of the following statements is true ?
Text Solution
|
- For the thermochemical equation, 2H(2(g)) + O(2(g)) rarr 2H(2)O(l) ,...
Text Solution
|
- The process is spontaneous at the given temperature, if
Text Solution
|
- The value of entropy of solar system is
Text Solution
|
- The ratio of heats liberated at 298 K from the combustion of one kg of...
Text Solution
|
- S + (3)/(2)O(2) rarr SO(3) + 2x kcal SO(2) + (1)/(2) O(2) rarr SO(3)...
Text Solution
|
- For one mole of NaCl(s) the lattice enthalpy is : Na(s) + (1)/(2)Cl(...
Text Solution
|
- An endothermic reaction is found to have +ve entropy change. The react...
Text Solution
|
- For an adiabatic change in a system, the condition which is applicable...
Text Solution
|