Text Solution
Verified by Experts
Topper's Solved these Questions
GENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
MODERN PUBLICATION|Exercise COMPETION FILE (Objective Questions) (Multiple Choice Question with only one correct answer)|25 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
MODERN PUBLICATION|Exercise COMPETION FILE (Objective Questions) (Multiple Choice Question from competitive examinations)|49 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
MODERN PUBLICATION|Exercise Revision Exercises(Short answer questions)|42 VideosELECTROCHEMISTRY
MODERN PUBLICATION|Exercise UNIT PRACTICE TEST|20 VideosHALOALKANES AND HALOARENES
MODERN PUBLICATION|Exercise UNIT PRACTICE TEST|11 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-GENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS-HOTS (Higher Order Thinking skills) (Advanced Level)
- Metals donot occur in nature as nitrates. Why ?
Text Solution
|
- Why the graphite rods in the extraction of aluminium from molten Al2 O...
Text Solution
|
- Why is zinc and not copper used for the recovery of metallic silver fr...
Text Solution
|
- Cinnabar (HgS) and galena (PbS) on roasting often give their respectiv...
Text Solution
|
- Graphite is used as anode but diamond is not. There exist free elect...
Text Solution
|
- Why is it advantageous to roast a sulphide ore to the oxide before red...
Text Solution
|
- Thermite process is quite useful for repairing broken parts of machine...
Text Solution
|
- The extraction of Au by leaching with NaCN both oxidation and reductio...
Text Solution
|
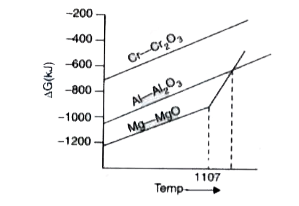
- A part of Ellingham diagram is shown below : (i) Will Cr2 O3 be red...
Text Solution
|
- Why partial roasting of sulphide ore is done in the metallurgy of copp...
Text Solution
|