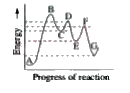A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL KINETICS
AAKASH SERIES|Exercise LEVEL-II(LECTURE SHEET EXERCISE-III MATCH THE FOLLOWING QUESTIONS)|3 VideosCHEMICAL KINETICS
AAKASH SERIES|Exercise LEVEL-II(LECTURE SHEET EXERCISE-IV INTEGER ANSWER TYPE QUESTIONS)|10 VideosCHEMICAL KINETICS
AAKASH SERIES|Exercise LEVEL-II(LECTURE SHEET EXERCISE-I SINGLE & ONE OR MORE THAN ONE CORRECT ANSWER)|50 VideosCHEMICAL KINETCS
AAKASH SERIES|Exercise EXERCISE - 3.2|45 VideosCHEMICAL THERMODYNAMICS
AAKASH SERIES|Exercise Additional Practice Exercise|54 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-CHEMICAL KINETICS-LEVEL-II(LECTURE SHEET EXERCISE-II LINKED COMPREHENSION TYPE QUESTIONS)
- A certain endothermic reaction: Ato Product, DeltaH=+ve proceeds ina s...
Text Solution
|
- A certain endothermic reaction: Ato Product, DeltaH=+ve proceeds ina s...
Text Solution
|
- A certain endothermic reaction: Ato Product, DeltaH=+ve proceeds ina s...
Text Solution
|
- A certain endothermic reaction: Ato Product, DeltaH=+ve proceeds ina s...
Text Solution
|
- A certain endothermic reaction: Ato Product, DeltaH=+ve proceeds ina s...
Text Solution
|
- A certain endothermic reaction: Ato Product, DeltaH=+ve proceeds ina s...
Text Solution
|
- The free energy profile for the transformation of A into G is shown be...
Text Solution
|
- The free energy profile for the transformation of A into G is shown be...
Text Solution
|
- The free energy profile for the transformation of A into G is shown be...
Text Solution
|