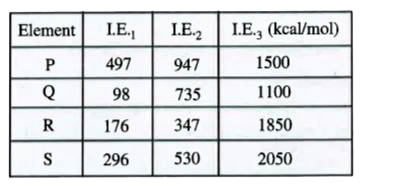
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
PERIODIC PROPERTIES
AAKASH SERIES|Exercise LEVEL-II (TYPE-I) (LECTURE SHEET-3 (MATCHING))|1 VideosPERIODIC PROPERTIES
AAKASH SERIES|Exercise LEVEL-II (TYPE-I) (PRACTICE SHEET-3 (MORE THAN ONE CORRECT ))|5 VideosPERIODIC PROPERTIES
AAKASH SERIES|Exercise LEVEL-II (TYPE-I) (LECTURE SHEET-3 (MORE THAN ONE CORRECT ))|5 VideosPERIODIC CLASSIFICATION
AAKASH SERIES|Exercise OBJECTIVE EXERCISE - 3 (RECENT AIPMT/NEET QUESTIONS)|14 VideosPERIODIC TABLE
AAKASH SERIES|Exercise EXERCISE ON PASSAGE|12 Videos
AAKASH SERIES-PERIODIC PROPERTIES-LEVEL-II (TYPE-I) (LECTURE SHEET-3 (COMPREHENSION))
- Consider metal (X) and non-metal (Y) react to form ionic compound. Giv...
Text Solution
|
- Consider metal (X) and non-metal (Y) react to form ionic compound. Giv...
Text Solution
|
- Consider metal (X) and non-metal (Y) react to form ionic compound. Giv...
Text Solution
|
- Ionization potential is the minimum amount of energy needed to remove ...
Text Solution
|
- Ionization potential is the minimum amount of energy needed to remove ...
Text Solution
|