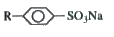A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SURFACE CHEMISTRY
AAKASH SERIES|Exercise LEVEL - II (LECTURE SHEET) (EXERCISE - II)(LINKED COMPREHENSION TYPE QUESTIONS)|5 VideosSURFACE CHEMISTRY
AAKASH SERIES|Exercise LEVEL - II (LECTURE SHEET) (EXERCISE - III)(MATCH THE FOLLOWING QUESTIONS)|3 VideosSURFACE CHEMISTRY
AAKASH SERIES|Exercise LEVEL - I (EXERCISE - II)(CATALYSIS)|3 VideosSTOICHIOMETRY
AAKASH SERIES|Exercise PRACTICE SHEET (ADVANCED) (INTEGER TYPE QUESTION)|2 VideosVA GROUP ELEMENTS
AAKASH SERIES|Exercise PRACTICE EXERCISE|51 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-SURFACE CHEMISTRY-LEVEL - II (LECTURE SHEET) (EXERCISE - I)(SINGLE & ONE OR MORE THAN ONE CORRECT ANSWERS)
- Oil soluble dye is mixed with water - in - oil emulsion then
Text Solution
|
- Oil soluble dye is mixed with emulsion and emulsion remains colourless...
Text Solution
|
- Non - ionogenic surfactants are
Text Solution
|
- Select correct properties of emulsions
Text Solution
|
- At CMC
Text Solution
|
- Above CMC, the surfactant molecules under go
Text Solution
|
- Which of the following is true in respect of adsorption
Text Solution
|
- In the adsorption of acetic acid by charcoal which of the following st...
Text Solution
|
- Calculate the surface area of a catalyst that adsorbs 10^3 cm^3 of N2 ...
Text Solution
|
- In Langmuir's model of adsorption of a gas on a solid surface
Text Solution
|
- Bredig arc method cannot be used to prepare colloidal solution of whic...
Text Solution
|
- Assosiated colloids
Text Solution
|
- The gold number of some colloids are given below {:("Colloid ","Gold...
Text Solution
|
- Artificial rain is caused by spray of
Text Solution
|
- Which one is an example of micelle system
Text Solution
|
- Which statement is correct ?
Text Solution
|
- Emulsifiers are generally
Text Solution
|
- According produces to the adsorption theory of catalysis, the speed of...
Text Solution
|
- Following are the events taking place to explain adsorption theory I...
Text Solution
|
- The effeciency of an enzyme in catalysing a reaction is due to its cap...
Text Solution
|