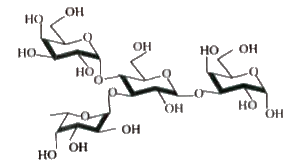
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CARBOHYDRATES
AAKASH SERIES|Exercise Practice Sheet-5|30 VideosCARBOHYDRATES
AAKASH SERIES|Exercise Practice Sheet-3|30 VideosBIOMOLECULES
AAKASH SERIES|Exercise OBJECTIVE EXERCISE-4 (ASSERTION (A) & REASON (R) TYPE QUESTIONS :)|84 VideosCARBOXYLIC ACIDS
AAKASH SERIES|Exercise PRACTICE SHEET-4 (Integer Type Questions)|9 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-CARBOHYDRATES-Practice Sheet-4
- The following carbohydrate is
Text Solution
|
- Which of the following statements are correct
Text Solution
|
- Similarities between D(+) Glucose and D(-) Fructose : correct statemen...
Text Solution
|
- Glucose does not react with
Text Solution
|
- Consider the following statements, correct statements
Text Solution
|
- The number of correct statements are
Text Solution
|
- Which two of the following aldohexoses give the same osazone derivativ...
Text Solution
|
- D underset((A))("Glucopyranose") underset("dry HCl")overset(MeOH)rarr ...
Text Solution
|
- D underset((A))("Glucopyranose") underset("dry HCl")overset(MeOH)rarr ...
Text Solution
|
- D underset((A))("Glucopyranose") underset("dry HCl")overset(MeOH)rarr ...
Text Solution
|
- Three carbohydrate molecules A, B and C were analysed by a scientist f...
Text Solution
|
- Three carbohydrate molecules A, B and C were analysed by a scientist f...
Text Solution
|
- Three carbohydrate molecules A, B and C were analysed by a scientist f...
Text Solution
|
- Match the following columns
Text Solution
|
- Match the following columns
Text Solution
|
- What is the ratio of possible aldohexoses and 2-ketohexoses
Text Solution
|
- Given below is a branched, substituded oligosaccharide in which 'All' ...
Text Solution
|
- When methyl -D- glucopyranoside is oxidized with periodic acid, how ma...
Text Solution
|
- The number of correct statements about the following compound (I)...
Text Solution
|
- Observe the following reaction and find out that how many number of re...
Text Solution
|