Similar Questions
Explore conceptually related problems
Recommended Questions
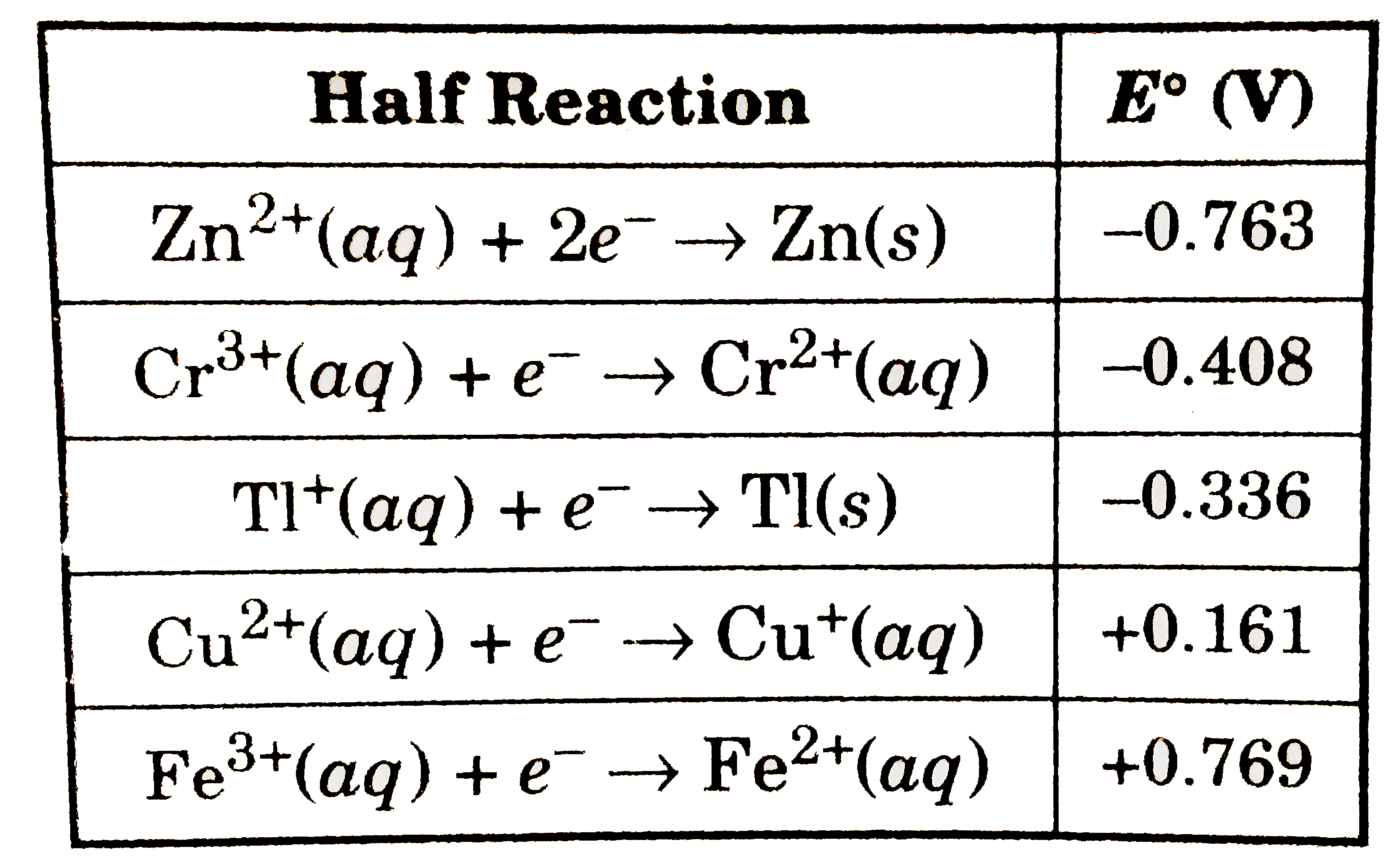
- Use the standard reduction potentials to find the standard cell potent...
Text Solution
|
- Consider the cell, Zn|Zn^(2+)(aq)(1.0M)||Cu^(2+)(aq)(1.0M)|Cu The stan...
Text Solution
|
- An electrochemical cell constructed for the reaction : Cu^(2+)(aq) + M...
Text Solution
|
- Ag^(+)(aq) + e^(-) rightarrow Ag(s) E^(@) = 0.80V Co^(2+)(aq) + 2e(-) ...
Text Solution
|
- Use the standard reduction potentials to find the standard cell potent...
Text Solution
|
- The standard electrode potential for Daniell cell is 1.1 V. Calculate ...
Text Solution
|
- डेनियल सेल के लिये मानक इलेक्ट्रोड विभव 1.1V है | निम्नलिखित अभिक्रिया...
Text Solution
|
- Zn^(2+)(aq)//Zn(s) अर्द्ध सैल के लिये मानक अपचयन विभव -0.76 वोल्ट है ।...
Text Solution
|
- Each of the following redox reaction occurs spontaneously. Zn(s)+Cu^...
Text Solution
|