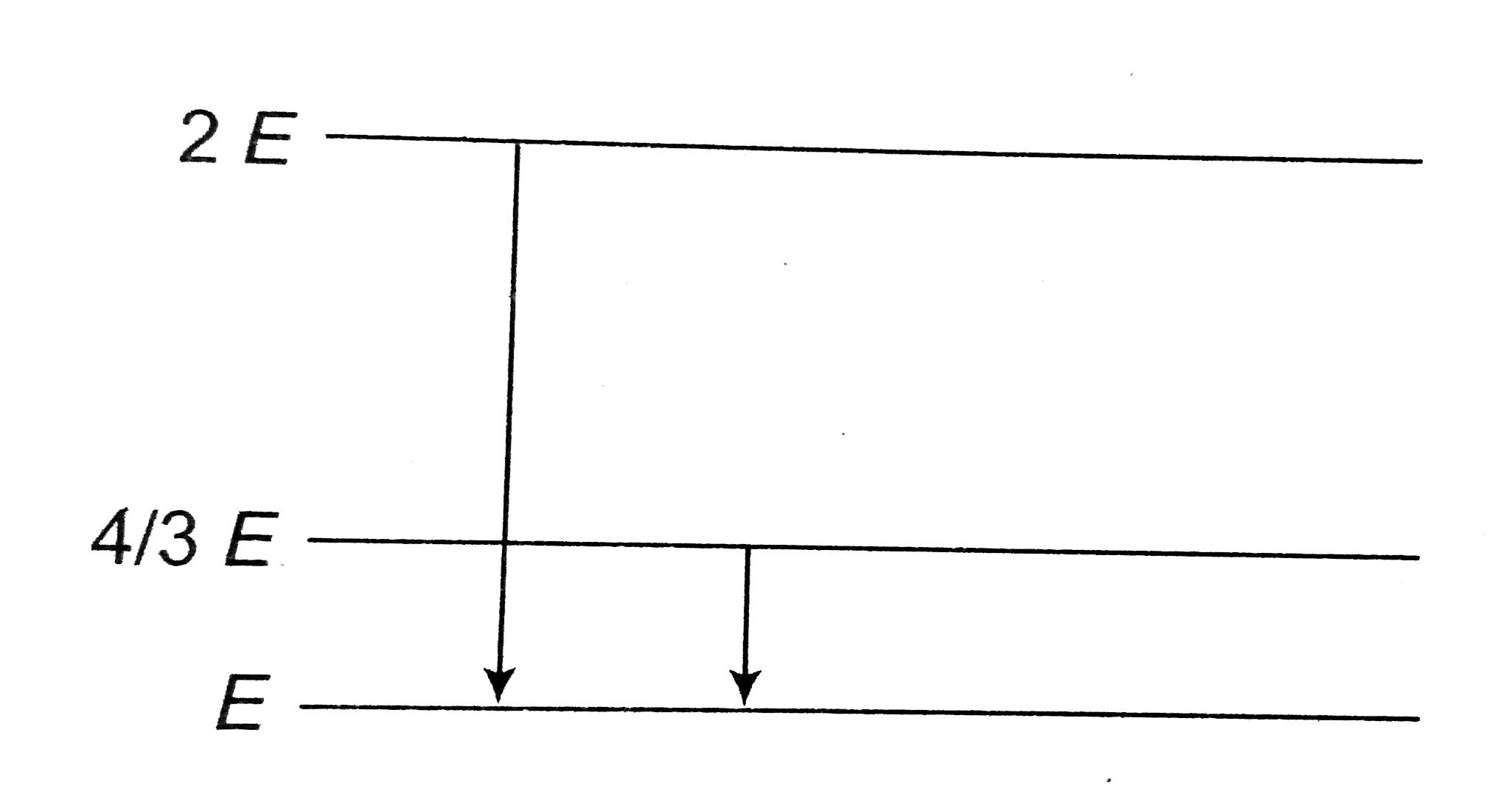
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DC PANDEY ENGLISH-ATOMS-MEDICAL ENTRANCES GALLERY
- The K(alpha) X-rays of moylydenum has a wavelength of 71xx10^(-12)m. I...
Text Solution
|
- Pick out the correct statement from the following
Text Solution
|
- Ligth emitted during the de excitation of electrons from n=3 to n=2, ...
Text Solution
|
- The binding energy of an electron in the ground state of He atom is ...
Text Solution
|
- The figure shows the energy level of certain atom. When the electron d...
Text Solution
|
- Spectrum of X-rays is
Text Solution
|
- The spectral series of the hydrogen spectrum that lies in the ultravio...
Text Solution
|
- The follwing diagram indicates the energy levels of a certain atom whe...
Text Solution
|
- As per the Bohr model, the minimum energy (in eV) required to remove a...
Text Solution
|
- If the series limit of Lyman series for hydrogen atom is equal to the ...
Text Solution
|
- The ratio of wavelenghts of photons emitted when hydrogen atom de-exci...
Text Solution
|
- An electron of a stationary hydrogen atom passes from the fifith energ...
Text Solution
|
- Assertion Balmer series lies in the visible region of electromagnetic...
Text Solution
|
- Assertion A beam of charged particles is employed in the treatment of ...
Text Solution
|
- In Bohr model of hydrogen atom, the force on the electron depends on t...
Text Solution
|
- The wavelength of K(a) line copper is 1.5Å. The ionisation energy of K...
Text Solution
|
- The wavelength of first line of Balmer series is 6563Å. The wavelength...
Text Solution
|
- The electron of a hydrogen atom revolves the proton in a circuit nth o...
Text Solution
|
- If an X-ray tube operates at the voltage of 10kV, find the ratio of th...
Text Solution
|
- Consider a hydrogen-like atom whose energy in nth excited state is giv...
Text Solution
|