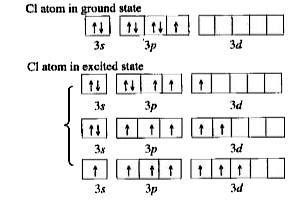
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ICSE-SAMPLE PAPER 2014-PART-II (SECTION -B)
- Write the formula of the following compounds : Triamminetriaquachrom...
Text Solution
|
- Write the formula of the following compounds : Potassiumhexacyanofer...
Text Solution
|
- Name the types of isomerism shown by the following pairs of compound [...
Text Solution
|
- Name the types of isomerism shown by the following pairs of compound [...
Text Solution
|
- For the complex ion of [Co(NH3)6]^(3+) State the hybridization of th...
Text Solution
|
- For the complex ion of [Co(NH3)6]^(3+) : State the magnetic nature ...
Text Solution
|
- Write balanced chemical equations for the following reactions : (i) ...
Text Solution
|
- Write balanced chemical equations for the following reactions : (i) ...
Text Solution
|
- Write balanced chemical equations for the following reactions : Sulp...
Text Solution
|
- Give reasons for the following: Zn^(2+) salts are white but Cu^(2+)...
Text Solution
|
- Give reasons for the following: Florine gives only one oxide but chl...
Text Solution
|
- How is potassium dichromate prepared from a sample of chromite ore ? G...
Text Solution
|
- For the molecule of IF(7) : (i) Draw the structure of the molecule ...
Text Solution
|
- For the molecule of IF(7) : (i) Draw the structure of the molecule ...
Text Solution
|
- For the molecule of IF(7) : (i) Draw the structure of the molecule ...
Text Solution
|