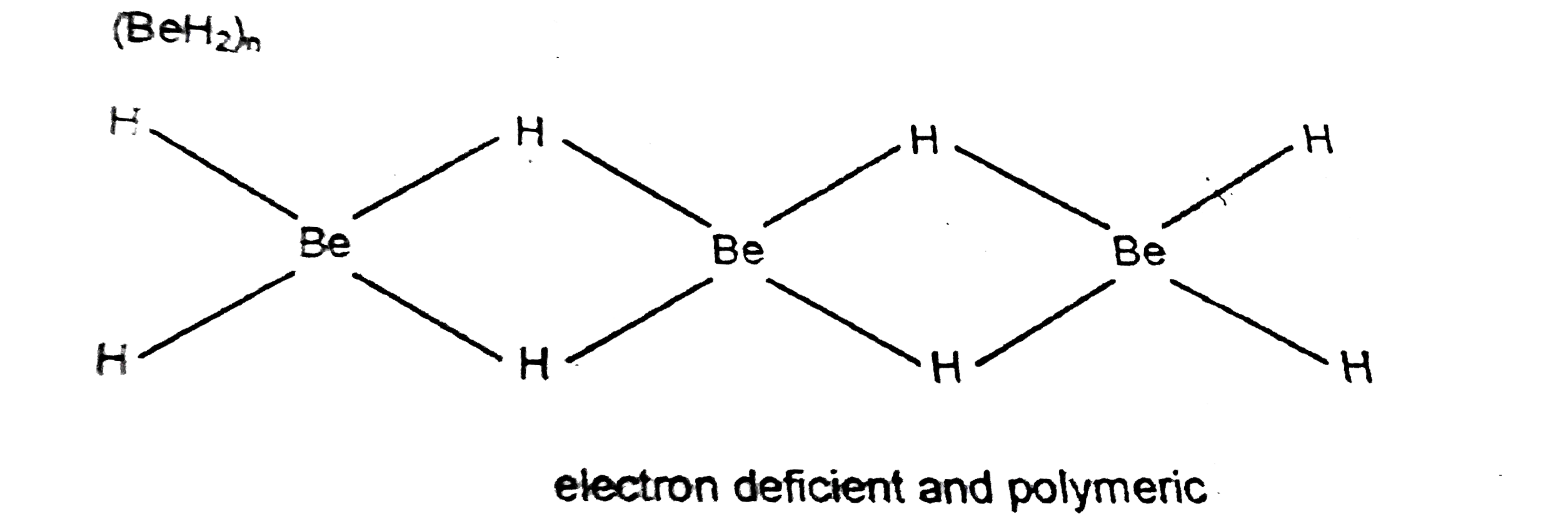
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-RANK BOOSTER-All Questions
- How many of the following nitrates of metal 'M' decompose on heating s...
Text Solution
|
- The decahydrate form of sodium carbonate i.e. washing soda on starding...
Text Solution
|
- Why Nitrogen does not form NCl(5) But phosphorous form PCl(5)? ?
Text Solution
|
- Which combination is correct for K metal
Text Solution
|
- Which combination is correct
Text Solution
|
- Aqueous solution of orthoboric acid can be titrated against sodium hyd...
Text Solution
|
- Select the correct statement about elements of group 15^(th)
Text Solution
|
- Single N-N bond is weaker than the single P-P bond. This is because ...
Text Solution
|
- Ammonia gas is not prepared by :
Text Solution
|
- Which of the following statement is correct ?
Text Solution
|
- 55 g of sodium chloride is dissolved in 100 g of water at 293 K. Find ...
Text Solution
|
- Hydrolysis of XeF4 with excess water gives :
Text Solution
|
- Consider the following statements and arrange in the order of true/fal...
Text Solution
|
- S(1): Argon is used in arc welding of metals or alloys to provide an i...
Text Solution
|
- Which of the following orders is correct? (1) SbH(3)gt NH(3) gt AsH(...
Text Solution
|
- Which of the following compounds is formed by addition of mineral acid...
Text Solution
|
- 35 g of sodium chloride is dissolved in 100 g of water at 293 K. Find ...
Text Solution
|
- B^(3+) cannot exist in aqueous solution because of its :
Text Solution
|
- The decrease in stability of higher oxidation state in p-block with in...
Text Solution
|
- 15 g of sodium chloride is dissolved in 100 g of water at 293 K. Find ...
Text Solution
|