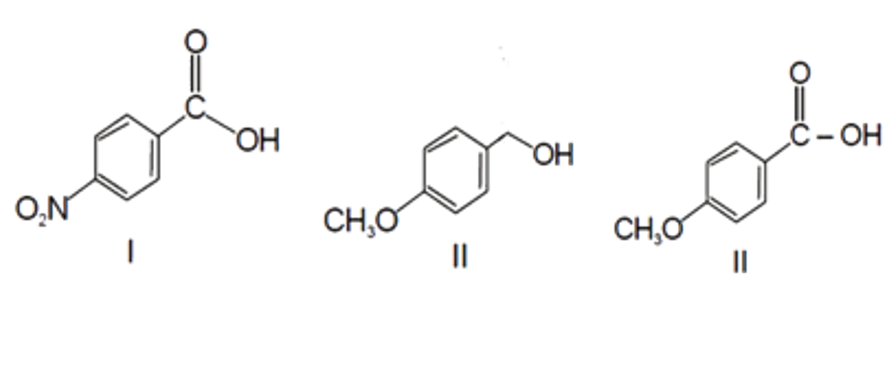
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Order of K(a) will be :
Text Solution
|
- Under the same reaction conditions, the intial concentration of 1.386 ...
Text Solution
|
- Under the same reaction condition, initial concentration of 1.386 mol ...
Text Solution
|
- Which of the following is not correct decreasing k(a) order .
Text Solution
|
- Order of K(a) of following acids is:
Text Solution
|
- K(a) of H(2)O(2) is of the order of
Text Solution
|
- Under the same reaction conditions, the intial concentration of 1.386 ...
Text Solution
|
- Arrange the following in decreasing order of their K(a) value :
Text Solution
|
- Under the same reaction conditions, initial concentration of 1.386" mo...
Text Solution
|