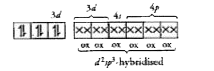
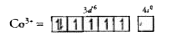Text Solution
Verified by Experts
Topper's Solved these Questions
COORDINATION COMPOUNDS
ARIHANT PUBLICATION|Exercise QUESTIONS FOR ASSESSMENT Part II Multiple Choice Type Questions 1 Mark |2 VideosCOORDINATION COMPOUNDS
ARIHANT PUBLICATION|Exercise QUESTIONS FOR ASSESSMENT Part II Very Short Answer Type Questions 1 Mark |5 VideosCOORDINATION COMPOUNDS
ARIHANT PUBLICATION|Exercise QUESTIONS FOR PRACTICE Part II Short Answer Type II Questions 3 Marks |12 VideosCHEMISTRY IN EVERYDAY LIFE
ARIHANT PUBLICATION|Exercise CHAPTER PRACTICE (LONG ANSWER TYPE QUESTIONS) |2 VideosD-BLOCK ELEMENTS
ARIHANT PUBLICATION|Exercise Chapter practice (Long answer type questions)|2 Videos
Similar Questions
Explore conceptually related problems
ARIHANT PUBLICATION-COORDINATION COMPOUNDS -QUESTIONS FOR PRACTICE Part II Long Answer Type Questions 7 Marks
- Discuss the nature of bonding in the following coordination entities o...
Text Solution
|
- Discuss the nature of bonding in the following coordination entities o...
Text Solution
|
- Discuss the nature of bonding in the following coordination entities o...
Text Solution
|
- Discuss the nature of bonding in the following coordination entities o...
Text Solution
|
- Write down t he IUPAC name for each of the following complexes and ind...
Text Solution
|
- Write down t he IUPAC name for each of the following complexes and ind...
Text Solution
|
- Write down t he IUPAC name for each of the following complexes and ind...
Text Solution
|
- Write down t he IUPAC name for each of the following complexes and ind...
Text Solution
|
- Write down t he IUPAC name for each of the following complexes and ind...
Text Solution
|