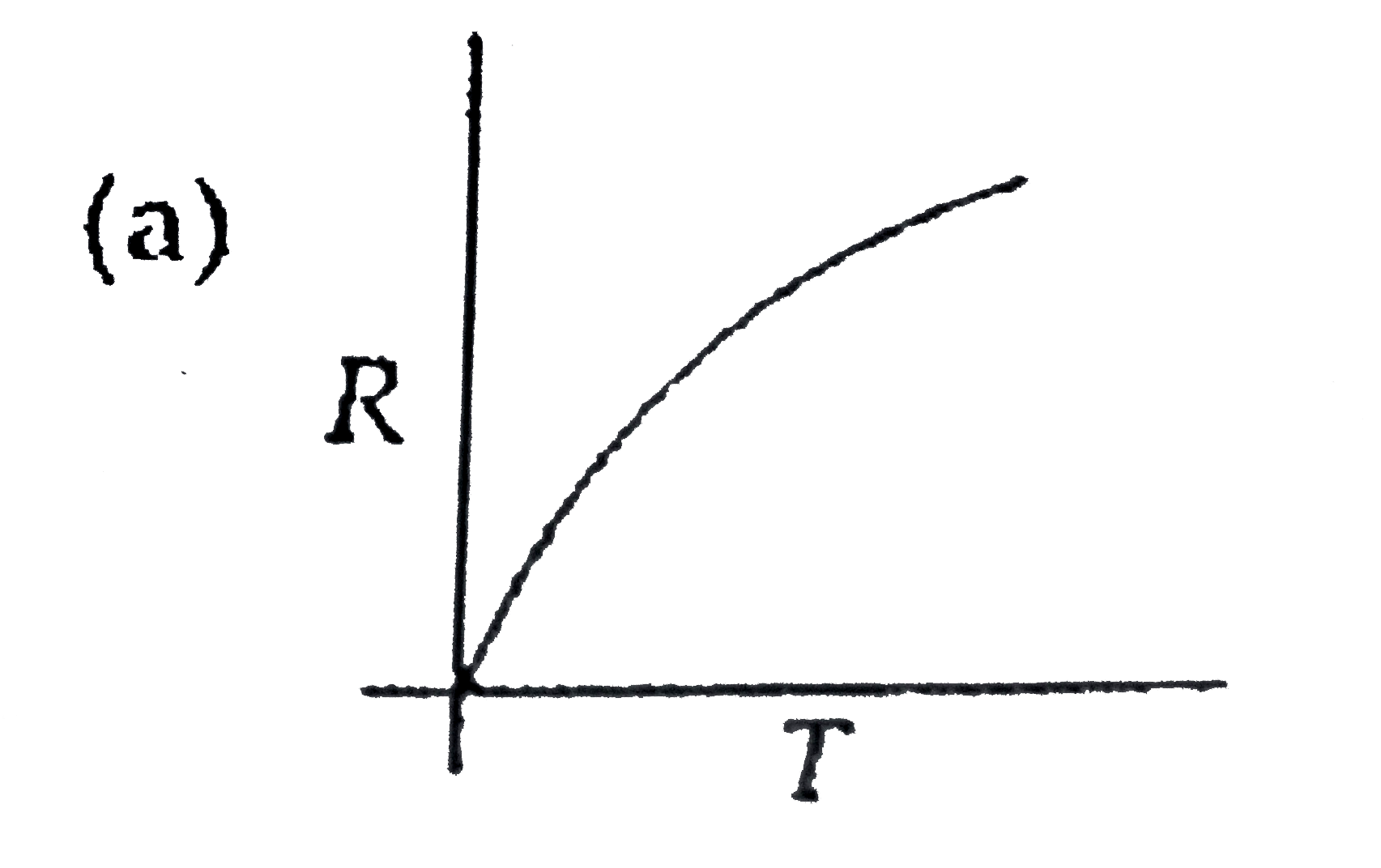
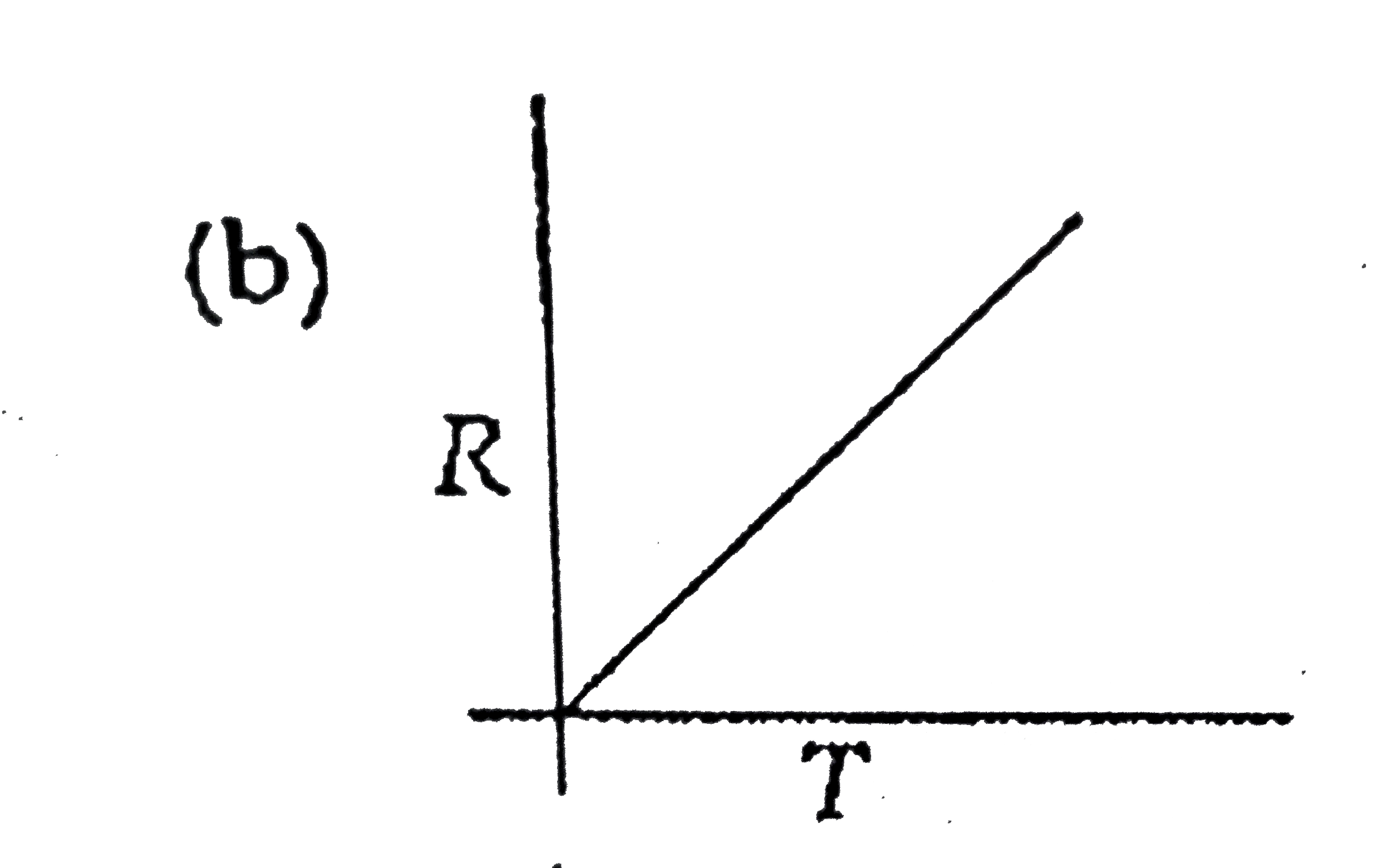
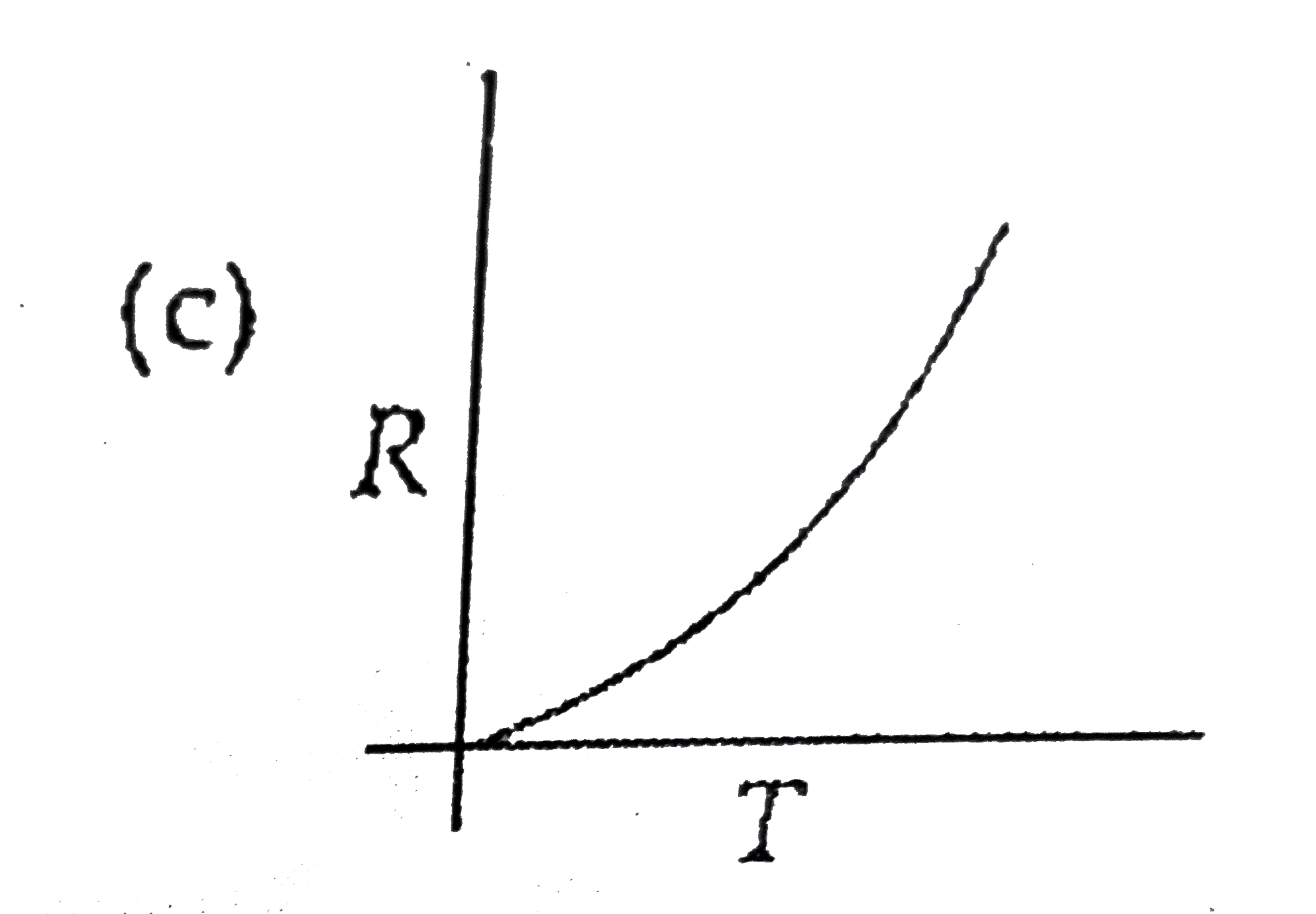
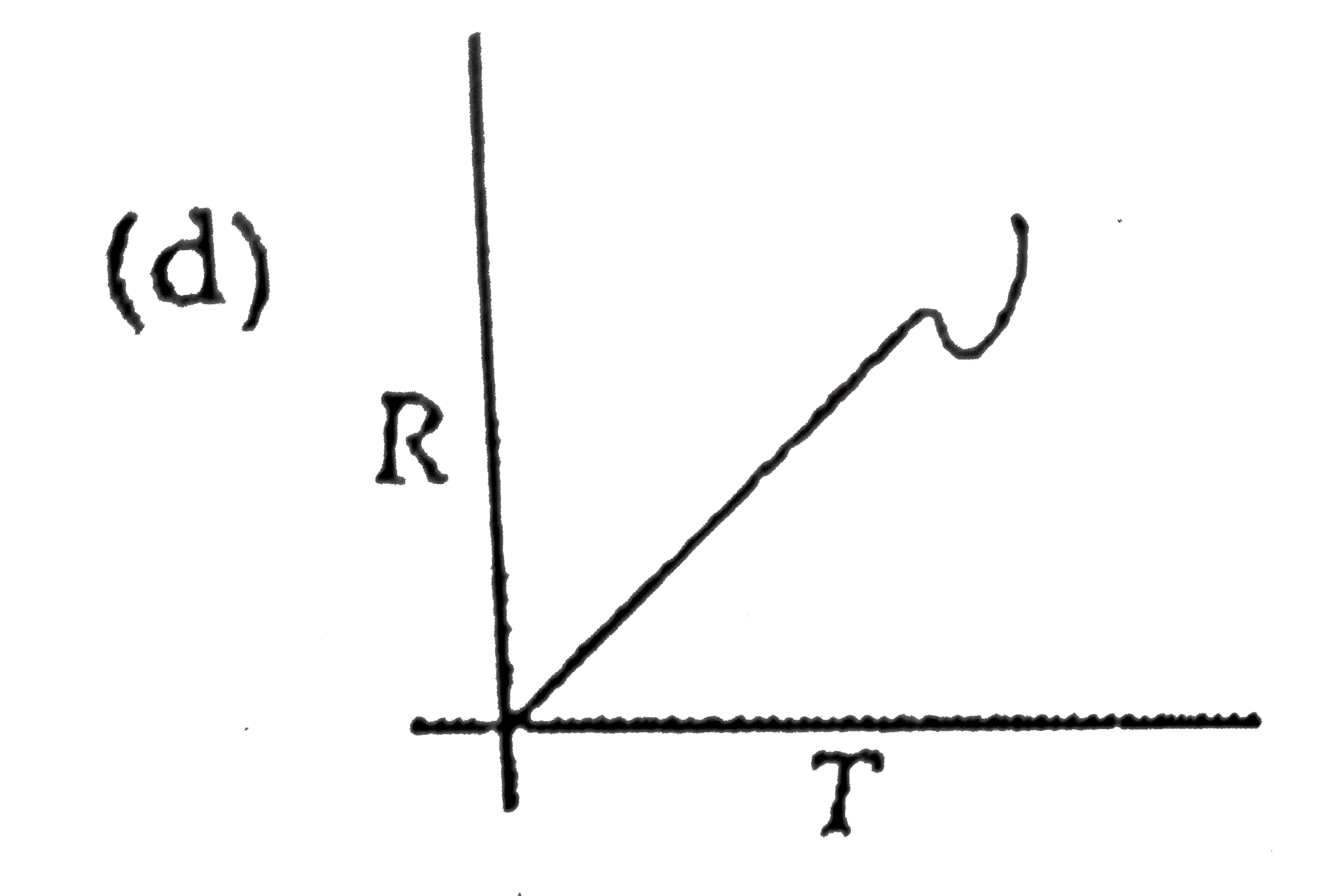A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which curve corresponds to the temperature dependance of the rate R ...
Text Solution
|
- Temperature Dependence Of The Rate Of A Reaction
Text Solution
|
- Which of the following graphs describes the typical dependence of the ...
Text Solution
|
- Which curve corresponds to the temperature dependance of the rate R of...
Text Solution
|
- The temperature dependence of the rate of a chemical reaction can be e...
Text Solution
|
- Why do reaction rates depend on temperature ? Explain.
Text Solution
|
- Write the energy distribution curve showing temperature dependence of ...
Text Solution
|
- Temperature Dependence Of Rate Of Reaction
Text Solution
|
- Temperature Dependence Of Rate Of Reaction
Text Solution
|