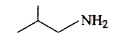
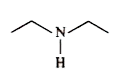
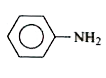
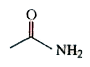
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following compounds will give isocyanide on reaction wi...
Text Solution
|
- Arrange following Amines for rate of reaction with CHCl(3)+KOH ?
Text Solution
|
- Which of the following will not gives positive test with CHCl(3) /KOH ...
Text Solution
|
- A compund reacts with CHCl(3) and KOH gives offensive smelling compoun...
Text Solution
|
- Which of the following compounds will give isocyanide test with CHCL(3...
Text Solution
|
- For the reaction of phenol with CHCl(3) in presence of KOH, the electr...
Text Solution
|
- Compound A on reduction gives B which on further reaction with CHCl(3)...
Text Solution
|
- Which of the following will not give positive test with CHCl(3)//KOH .
Text Solution
|
- The compound which does not give foul smell when heated with CHCl(3) a...
Text Solution
|