Similar Questions
Explore conceptually related problems
Recommended Questions
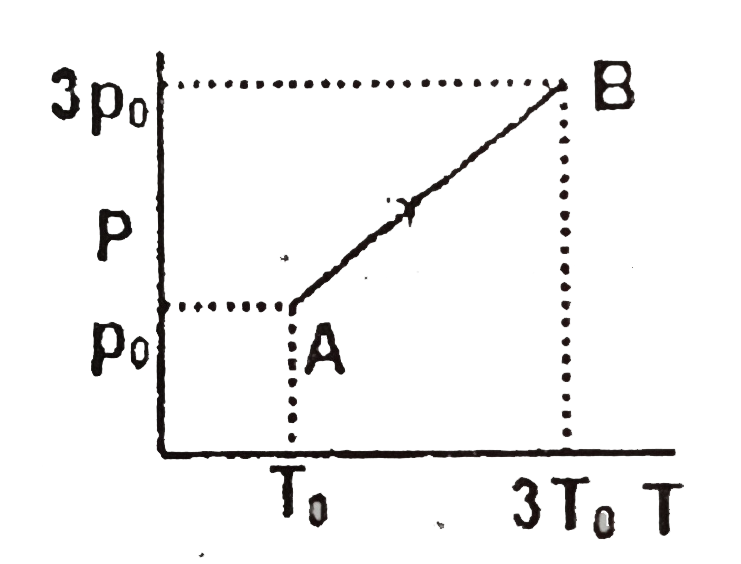
- Two moles of a monoatomic ideal gas undergoes a process AB as shown in...
Text Solution
|
- An ideal monoatomic gas undergoes the process AB as shown in the figur...
Text Solution
|
- One mole of an ideal monoatomic gas undergoes a process as shown in th...
Text Solution
|
- An ideal monoatomic gas undergoes a cyclic process ABCA as shown in th...
Text Solution
|
- V-T graph of a process of monoatomic ideal gas is shown in figure. ...
Text Solution
|
- Two moles of an ideal monoatomic gas undergoes a cyclic process ABCA a...
Text Solution
|
- Two moles of an ideal monoatomic gas undergoes a cyclic process ABCA a...
Text Solution
|
- An ideal gas system undergoes an isothermal process, then the work don...
Text Solution
|
- Three moles of an ideal gas undergo a cyclic process shown in figure....
Text Solution
|