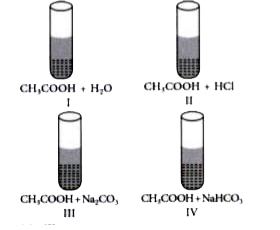
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MTG IIT JEE FOUNDATION-FOOTSTEPS TOWARDS (NEET) -MCQ
- Which of the following metals is protected even by a layer of its oxid...
Text Solution
|
- When washing soda is treated with hydrochloric acid, it gives off a co...
Text Solution
|
- In which of the following test tubes, the gas on evolution extinguishe...
Text Solution
|
- On moving from left to right in periodic table, which of the following...
Text Solution
|
- One of the following compound is not ionic in nature. This compound is
Text Solution
|
- A student added a few drops of the universal indicator to a solution o...
Text Solution
|
- The atomic numbers of four elements are given below: Which of t...
Text Solution
|
- Four solutions labelled as A, B, C and Dhave pH values 1,8, 3 and 13 r...
Text Solution
|
- Phosphorus(V) chloride dissolves in water to form phosphoric acid, H(3...
Text Solution
|
- Which of the following pairs of compounds of carbon will undergo combu...
Text Solution
|
- Which of the following combination of elements belong to the same grou...
Text Solution
|
- A student was given four unknown colourless samples labelled A, B, C a...
Text Solution
|
- On burning magnesium ribbon in air, it is observed that
Text Solution
|
- Consider the following table : The formula of compound formed bet...
Text Solution
|
- Which of the following is correct order of the reactivity of the metal...
Text Solution
|
- A student takes about 5 mL distilled water in four test tubes marked P...
Text Solution
|
- In the reaction, Br(2) +2I^(-) to 2Br^(-) +I(2), the oxidising agent ...
Text Solution
|
- An experiment was carried out using the apparatus shown below: ...
Text Solution
|
- Which of the following elements is not a metal?
Text Solution
|
- When 1.0 mol of compound Q is burnt in excess oxygen, 2.0 mol of carbo...
Text Solution
|