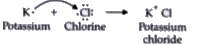Text Solution
Verified by Experts
Topper's Solved these Questions
PERIODIC CLASSIFICATION OF ELEMENTS
OSWAL PUBLICATION|Exercise NCERT EXEMPLAR (LONG ANSWER QUESTIONS)|36 VideosPERIODIC CLASSIFICATION OF ELEMENTS
OSWAL PUBLICATION|Exercise BOARD CORNER (Short Answer Type Questions) |12 VideosPERIODIC CLASSIFICATION OF ELEMENTS
OSWAL PUBLICATION|Exercise NCERT EXEMPLAR (MULTIPLE CHOICE QUESTIONS)|26 VideosOLYMPIAD 2019 - 20
OSWAL PUBLICATION|Exercise CHEMISTRY QUESTIONS (Is Matter Around us Pure)|1 VideosSAMPLE PAPER 1
OSWAL PUBLICATION|Exercise QUESTION BANK|9 Videos
Similar Questions
Explore conceptually related problems
OSWAL PUBLICATION-PERIODIC CLASSIFICATION OF ELEMENTS -NCERT EXEMPLAR (SHORT ANSWER QUESTIONS)
- Write the formulae of chlorides of Eka-Silicon and Eka-aluminium, the ...
Text Solution
|
- Three elements A, B and C have 3,4 and 2 electrons respectively in the...
Text Solution
|
- If an element X is placed in group 14, what will be the formula and th...
Text Solution
|
- Compare the radii of two species X and Y. Give reasons for your answer...
Text Solution
|
- Arrange the following elements in increasing order of their atomic rad...
Text Solution
|
- Arrange the following elements in increasing order of their atomic rad...
Text Solution
|
- Identify and name the metals whose electronic configuration is given...
Text Solution
|
- Identify and name the metal whose electronic configuration are given b...
Text Solution
|
- Identify and name the element whose electronic configuration are given...
Text Solution
|
- Identify and name the metal whose electronic configuration is given b...
Text Solution
|
- Write the formula of the product formed when the element A (atomic num...
Text Solution
|
- Arrange the following elements in the increasing order of their metall...
Text Solution
|
- Identify, the elements with the following property . An element whic...
Text Solution
|
- Identify, the elements with the following property The metal which i...
Text Solution
|
- Identify, the elements with the following property The metal which e...
Text Solution
|
- Properties of the elements are given below. Where would you locate the...
Text Solution
|
- Properties of the elements are given below. Where would you locate the...
Text Solution
|
- Properties of the elements are given below. Where would you locate the...
Text Solution
|
- Properties of the elements are given below. Where would you locate the...
Text Solution
|
- Properties of the elements are given below. Where would you locate the...
Text Solution
|