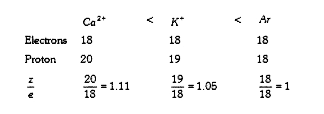A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CLASSFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
ERRORLESS|Exercise Assertion & Reason |14 VideosCLASSFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
ERRORLESS|Exercise NCERT BASED QUESTIONS (Valency and Oxidation State)|25 VideosCHEMICAL EQUILIBRIUM
ERRORLESS|Exercise ASSERTION & REASON |12 VideosENVIRONMENTAL CHEMISTRY
ERRORLESS|Exercise ASSERTION & REASON|7 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-CLASSFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES -PAST YEARS QUESTIONS
- Which of the following orders of ionic radii is correctly represented ...
Text Solution
|
- Which of the following statement concerning lanthanides elements is fa...
Text Solution
|
- The species Ar,K^(+) and Ca^(2+) contain the same number of electrons....
Text Solution
|
- which of the following ions is the smallest in size?
Text Solution
|
- Among the following which has the highest cation to anion size ratio ...
Text Solution
|
- Which of the following element has maximum, first ionisation potential...
Text Solution
|
- Which of the following gaseous atoms has highest value of IE?
Text Solution
|
- Hydrogen has high ionization energy than alkali metals, due to its:
Text Solution
|
- The first ionisation potentials (eV) of Be and B respectively are
Text Solution
|
- Arrange S, P and As in order of increasing ionisation energy.
Text Solution
|
- Among the following options , the sequence of increasing first ionisat...
Text Solution
|
- Which of the following has minimum ionisation energy
Text Solution
|
- Which has the highest second ionisation potenitial?
Text Solution
|
- Which of the following order of ionisation energy is correct?
Text Solution
|
- The second ionisation potential of an element M is the energy required...
Text Solution
|
- Which of the following transitions involves maximum amount of energy?
Text Solution
|
- For the second period elements the correct increasing order of first i...
Text Solution
|
- For the electron affinity of halogens (with -ve sign), which of the fo...
Text Solution
|
- Which one of the following arrangements represents the correct order o...
Text Solution
|
- The formation of oxide ion O^(2-)(g) from oxygen atom requires first a...
Text Solution
|