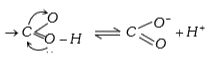A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Carboxylic acids are easily ionised. The main reason of this statement
Text Solution
|
- Carboxylic acids undergo ionisation due to
Text Solution
|
- What is the main reason for the fact that carboxylic acids can undergo...
Text Solution
|
- Mineral acids are stronger acids than carboxylic acids because (i) m...
Text Solution
|
- Carboxylic acids are easily ionised. The main reason of this statement
Text Solution
|
- What is the main reason for the fact that carboxylic acid can undergo ...
Text Solution
|
- What is the main reason for the fact that carboxylic acids can undergo...
Text Solution
|
- Assertion: Compounds containing -CHO group are easily oxidized to corr...
Text Solution
|
- कार्बोंक्सिलिक अम्ल आसानी से आयनीकृत होते हैं। इस तथ्य का मुख्य कारण ह...
Text Solution
|