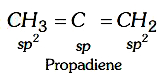A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- What is meant by hybridisation ? Compound CH(2)=C=CH(2) contains sp or...
Text Solution
|
- What is meant by hybridisation ? Compound CH(2)=C=CH(2) contains sp or...
Text Solution
|
- Assertion (A) Formaldehyde is a planar molecule. Reason (R) It contain...
Text Solution
|
- In the organic compound CH(2)= CH - CH(2) - CH(2) - C -= CH , the pair...
Text Solution
|
- Assertion: Formaldehyde is a planar molecule. Reason: It contains sp^(...
Text Solution
|
- What will be the shape of a hydrocarbon molecule containing two sp^(2)...
Text Solution
|
- In the following compound, the number of 'sp' hybridised carbon is CH(...
Text Solution
|
- The shape of the molecule containing only sp^(2) - hybridised carbon a...
Text Solution
|
- Consider the given statement about the molecule 1. Three carbon a...
Text Solution
|