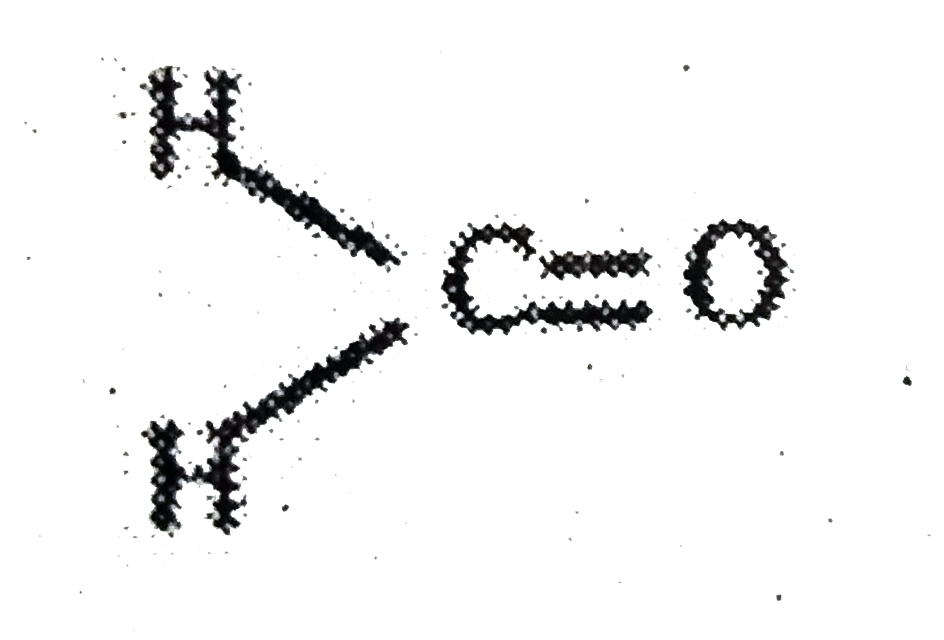
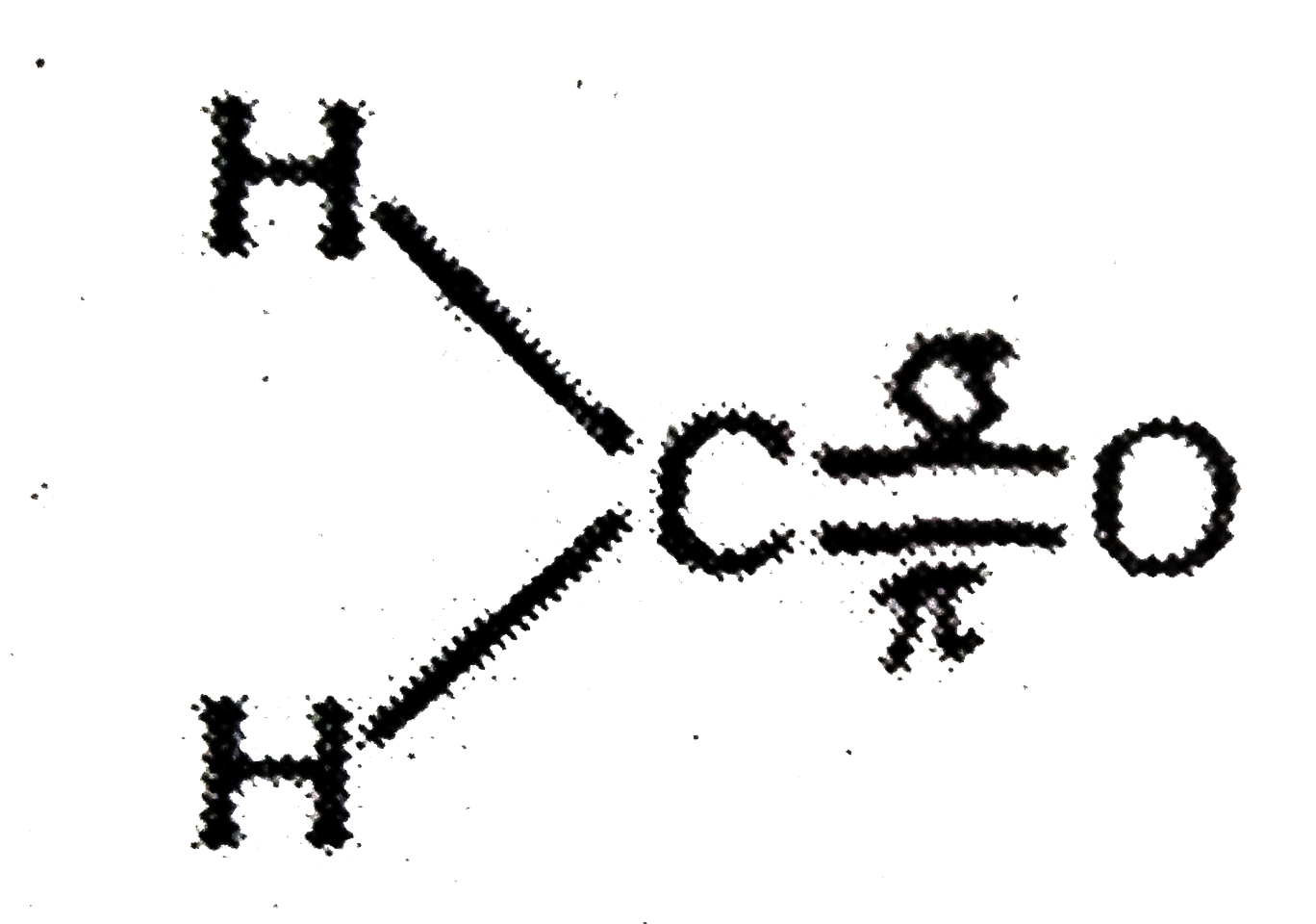
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
AIIMS PREVIOUS YEAR PAPERS-AIIMS 1999-CHEMISTRY
- The protons and neutrons in the nuclei of atoms undergo inter-conversi...
Text Solution
|
- 18 carat gold contains
Text Solution
|
- Liquid hydrogen is being seriously considered as automobile fuel. It i...
Text Solution
|
- One a.m.u. is equal to
Text Solution
|
- The normality of conc. HCl used in the laboratory is
Text Solution
|
- Which of the following will have least hindered rotation about carbon-...
Text Solution
|
- Units for the rate constant k of the zero order rate equation are
Text Solution
|
- Proteins are characterized by the linkage
Text Solution
|
- Which compound does not dissolve in hot dil. HNO3?
Text Solution
|
- Which of the following ions is not isoelectronic with the other three ...
Text Solution
|
- Assertion: Both 12 g. of carbon and 27 g. of aluminium will have 6.02x...
Text Solution
|
- Assertion:Sucrose is sweetest in taste Reason : Sucrose is converted...
Text Solution
|
- Assertion (A): Potassium cannot be obtained by the electrolysis of use...
Text Solution
|
- Assertion: Electrons are ejected from a certain metal when either blue...
Text Solution
|
- Assertion: Cyclobutane is less stable than cyclopentane Reason : T...
Text Solution
|
- Assertion:Benzoyl chloride is used for the preparation of derivative o...
Text Solution
|
- Assertion:In formaldehyde, all the four atoms are in the same plane ...
Text Solution
|
- Assertion (A) : A spectral line will be seen for 2p(x)-2p(y) transitio...
Text Solution
|
- Assertion: lt is very difficult to subject vinyl chloride to nucleophi...
Text Solution
|
- Statement-I : The configuration of B atom cannot be 1s^(2) 2s^(3). ...
Text Solution
|