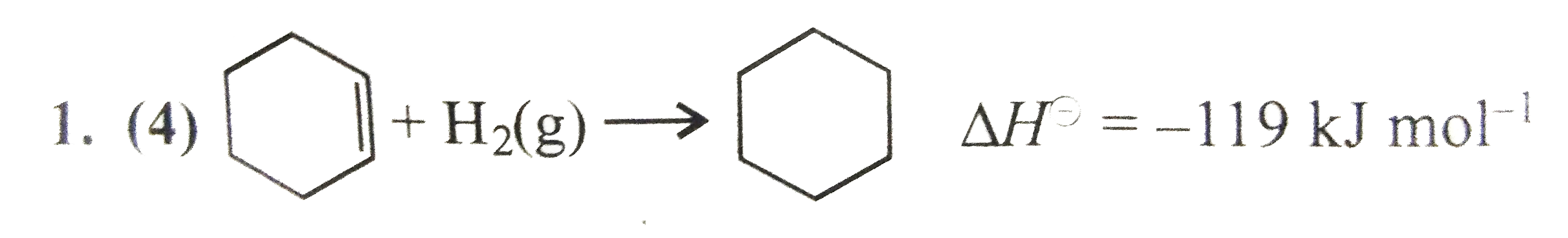
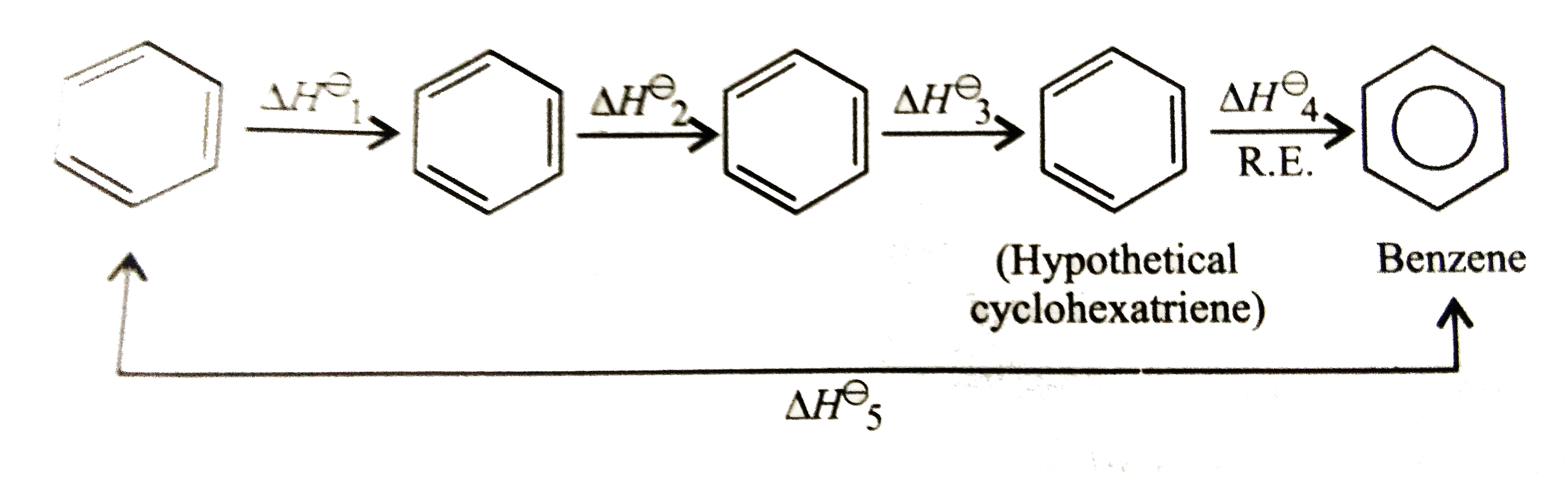
Text Solution
Verified by Experts
Topper's Solved these Questions
THERMODYNAMICS
CENGAGE CHEMISTRY|Exercise Exercises (Fill In The Blanks)|52 VideosTHERMODYNAMICS
CENGAGE CHEMISTRY|Exercise Exercises (True/False)|32 VideosTHERMODYNAMICS
CENGAGE CHEMISTRY|Exercise Exercises (Assertion-Reasoning)|23 VideosSTOICHIOMETRY
CENGAGE CHEMISTRY|Exercise Archives Subjective|33 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-THERMODYNAMICS-Exercises (Interger)
- Delta(f)H^(Theta) of Cyclohexene (l) and benzene at 25^(@)C is -156 an...
Text Solution
|
- Bond disociation enegry of XY,X(2) and Y(2) (all diatomic molecules) a...
Text Solution
|
- The polymerisation of propene to linear polypropene is represented by ...
Text Solution
|
- Delta(f)H^(Theta) of hypothetical MgCI is -125 kJ mol^(-1) and for MgC...
Text Solution
|
- The lattice energy of solid KCI is 181 kcal mol^(-1) and the enthalpy ...
Text Solution
|
- A heated irron block at 127^(@)C loses 300J of heat to the surrounding...
Text Solution
|
- Amongst the following, the total number of reactions/processes in whic...
Text Solution
|
- Amongst the following, the total number of physical properties which a...
Text Solution
|
- Amongst the following in above mention the total number of intensive p...
Text Solution
|
- Calculate the entropy change accompanying the following change of stat...
Text Solution
|