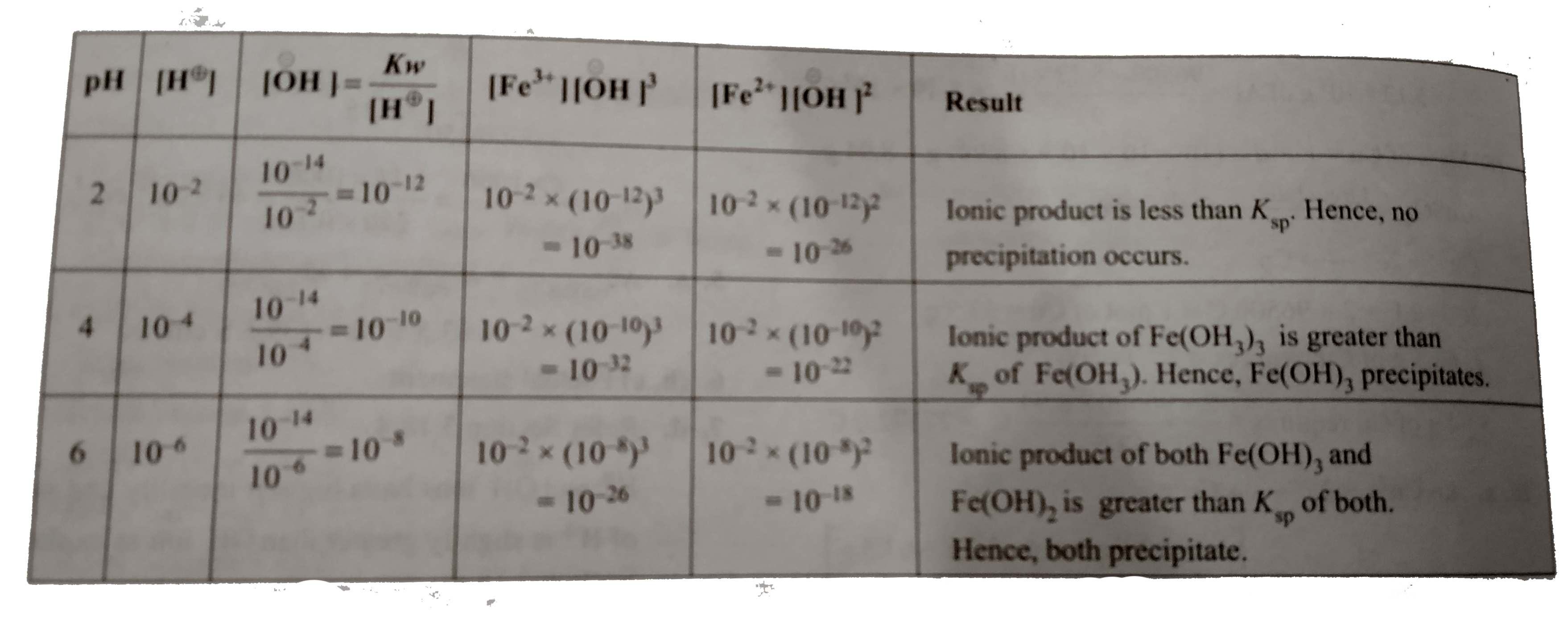
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISTRY
CENGAGE CHEMISTRY|Exercise Exercisemultiple Correct Ansers|53 VideosELECTROCHEMISTRY
CENGAGE CHEMISTRY|Exercise Exercises Ingle Correct|178 VideosELECTROCHEMISTRY
CENGAGE CHEMISTRY|Exercise Ex 3.3 (Objective)|10 VideosD AND F BLOCK ELEMENTS
CENGAGE CHEMISTRY|Exercise Archives Subjective|29 VideosGENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTS
CENGAGE CHEMISTRY|Exercise Archives (Subjective)|14 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-ELECTROCHEMISTRY-Exercise(Linked Comprehension )
- An aqueous solution containing 0.1M Fe^(3+) and 0.01 M Fe^(2+) was tit...
Text Solution
|
- An aqueous solution containing 0.1M Fe^(3+) and 0.01 M Fe^(2+) was tit...
Text Solution
|
- An aqueous solution containing 0.1M Fe^(3+) and 0.01 M Fe^(2+) was tit...
Text Solution
|
- Calculate Delta(r)G^(c-) of the reaction : Ag^(o+)(aq)+Cl^(c-)(aq) r...
Text Solution
|
- E^(c-).(cell) of the reaction above in Question is
Text Solution
|
- K(sp) of AgCl is
Text Solution
|
- 6.537 xx 10^(-2)f of metallic Zn was added to 100 mL of saturated sol...
Text Solution
|
- Calculate the number of moless of Ag formed above in Question.
Text Solution
|
- The magnitude ( but not the sign ) of the standard reduction potential...
Text Solution
|
- The magnitude ( but not the sign ) of the standard reduction potential...
Text Solution
|
- The magnitude ( but not the sign ) of the standard reduction potential...
Text Solution
|
- A volataic cell consists of an electode of solide silver immerse in a ...
Text Solution
|
- A volataic cell consists of an electode of solide silver immerse in a ...
Text Solution
|
- Fuel cells : Fuel cells are galvanic cells in which the chemical energ...
Text Solution
|
- Fuel cells : Fuel cells are galvanic cells in which the chemical energ...
Text Solution
|
- Fuel cells : Fuel cells are galvanic cells in which the chemical energ...
Text Solution
|
- Fuel cells : Fuel cells are galvanic cells in which the chemical energ...
Text Solution
|
- A conductivity cell is used to measure the conductance of electrolyte ...
Text Solution
|
- A conductivity cell is used to measure the conductance of electrolyte ...
Text Solution
|
- Breathalyzer is used to detect the alcohol content in the suspected dr...
Text Solution
|