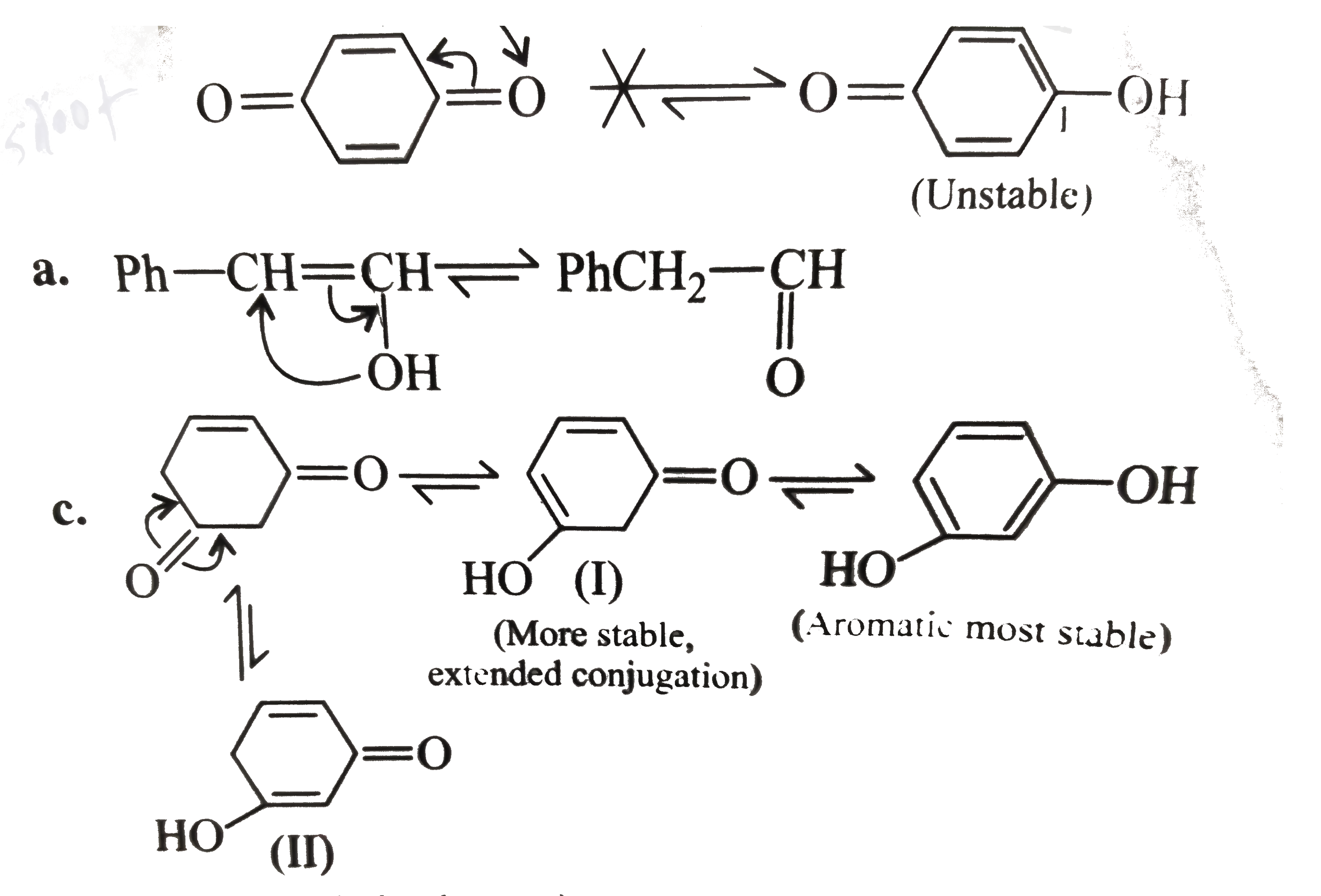
(I) (i),(b,d)
(a) and (c ) do not have `alpha-H` atom, so they do not show tautomerism.
(ii) (a,c,d)
(b) does not show tautomerism, since two double bonds would be in cumulative position, which is unstable. `C-2` is `sp`-hybridised and the linear structural unit cannot be bridged with only two `C` atoms but can be bridge with four `C` atoms and hence it does not show tautomerism.
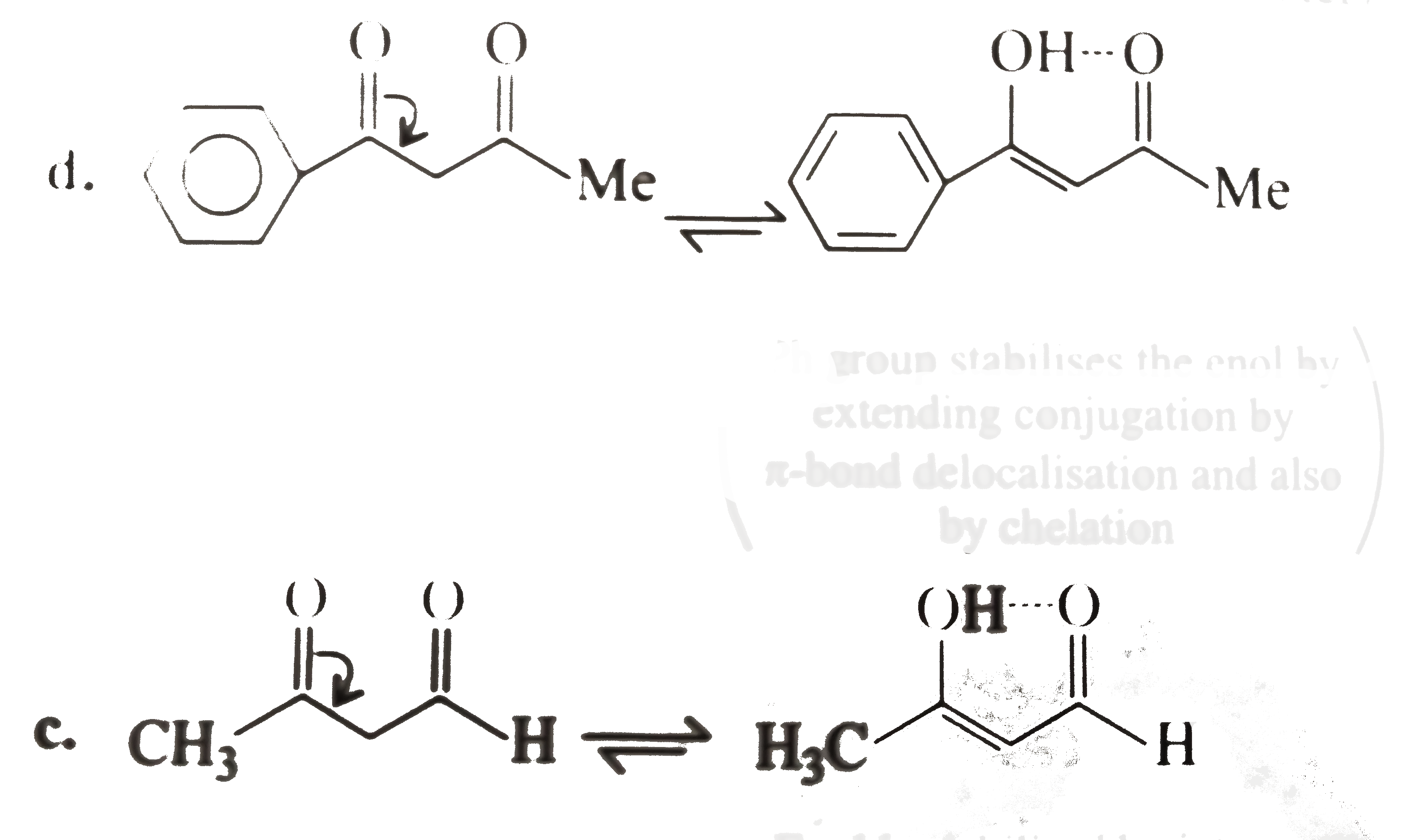
(II) I=i. `d gt a gt b gt c("diketone" gt "Ketone-aldehyde" gt "Ketone" gt "aldehyde")`
ii.`d gt c gt b gt a (80% gt 76% gt 7.7% gt 1%)("diketone with (ph) group" gt "diketone" gt "Keto easter" gt "diester")`
d. Esters do not show tautomerism due to cross conjugated resonance structrues, which descrease the ability of `(C=O)` group of easter in stabilising the enol from.
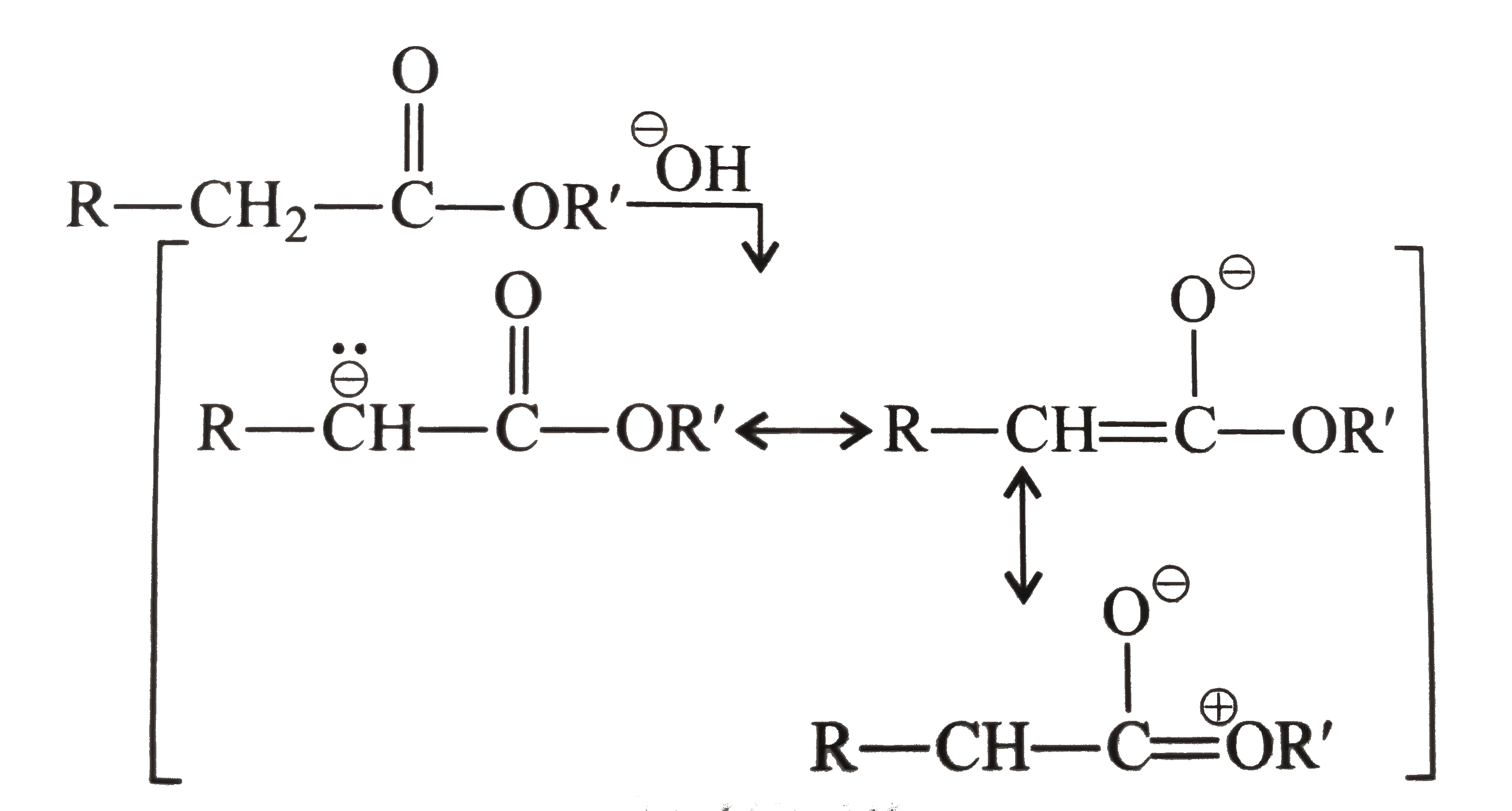
So diethyl malonate does not show tautomerism.
(iii)`b gt a gt c`
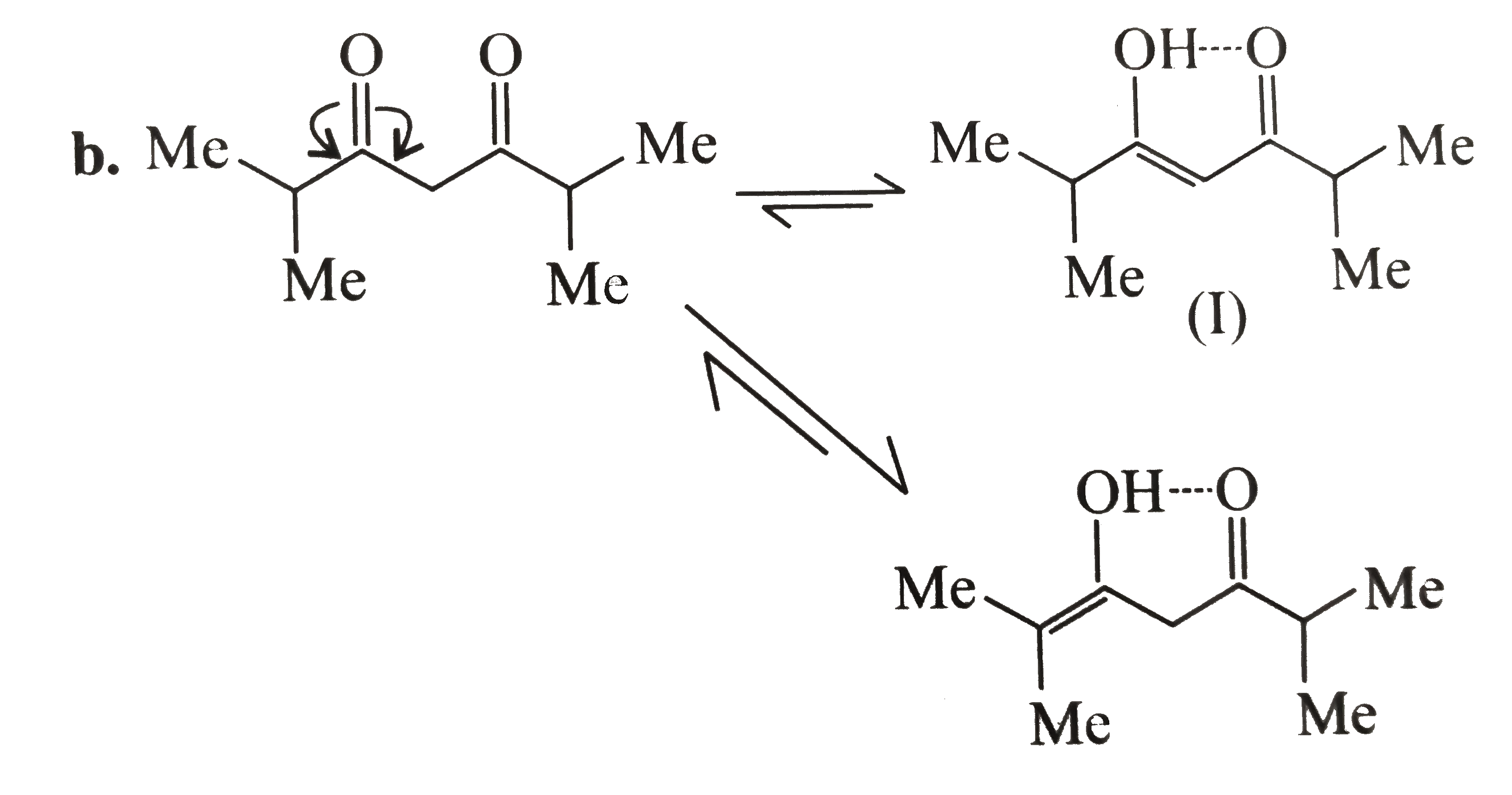
(I) is more stable than (II), since (II) is more stained `5`-membered ring joining the `(C==C)` bond.
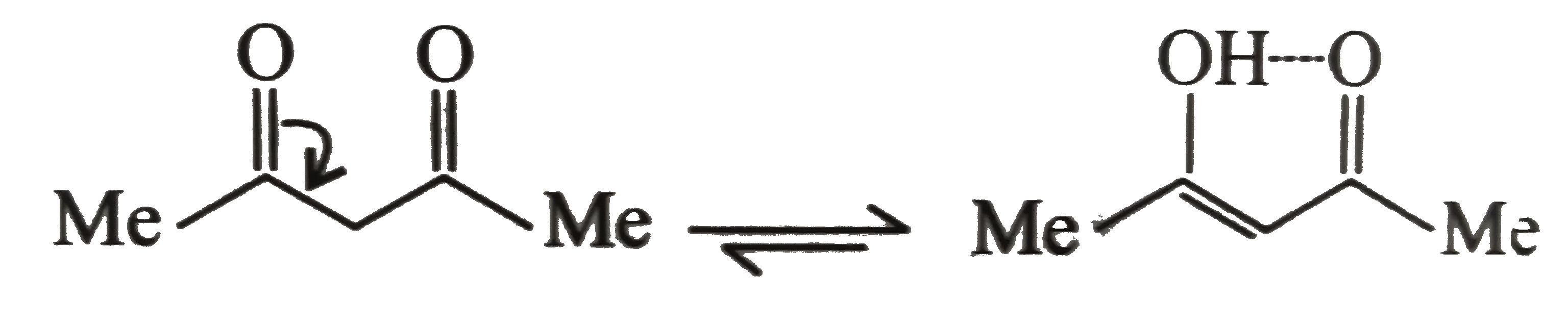
Both (b) and (a) are stabilised by intramolecular `H`-bonding. But (b) shows more enol content than (a) since (b) is more substitued alkene.
In (c ) there is only one `alpha-H` atom and its acidity is decreased by `bar (e)`-donating (+I effect of )isopropyl group It shows less enol form.
III. Effect of solvent on enol content `c gt d gt b gt a`. Benzene (non-polar) `gt` acetone (slightly polar) `gt` methanol (polar, protic solvent) `gt H_(2)O` (highly polar, protic solvent)