I
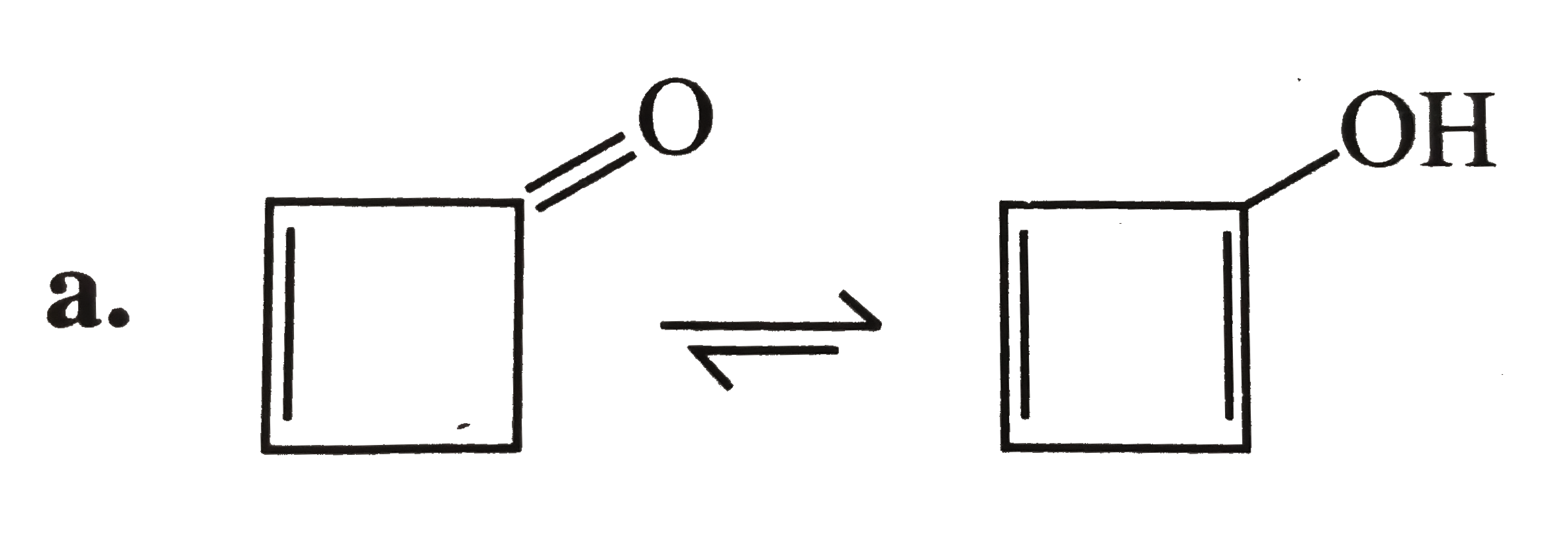
Doesn't enolise because its enol is a flat ring with `4n pi bar e(i.e., 4pi bar e' s,n=1)` and is antiaromatic
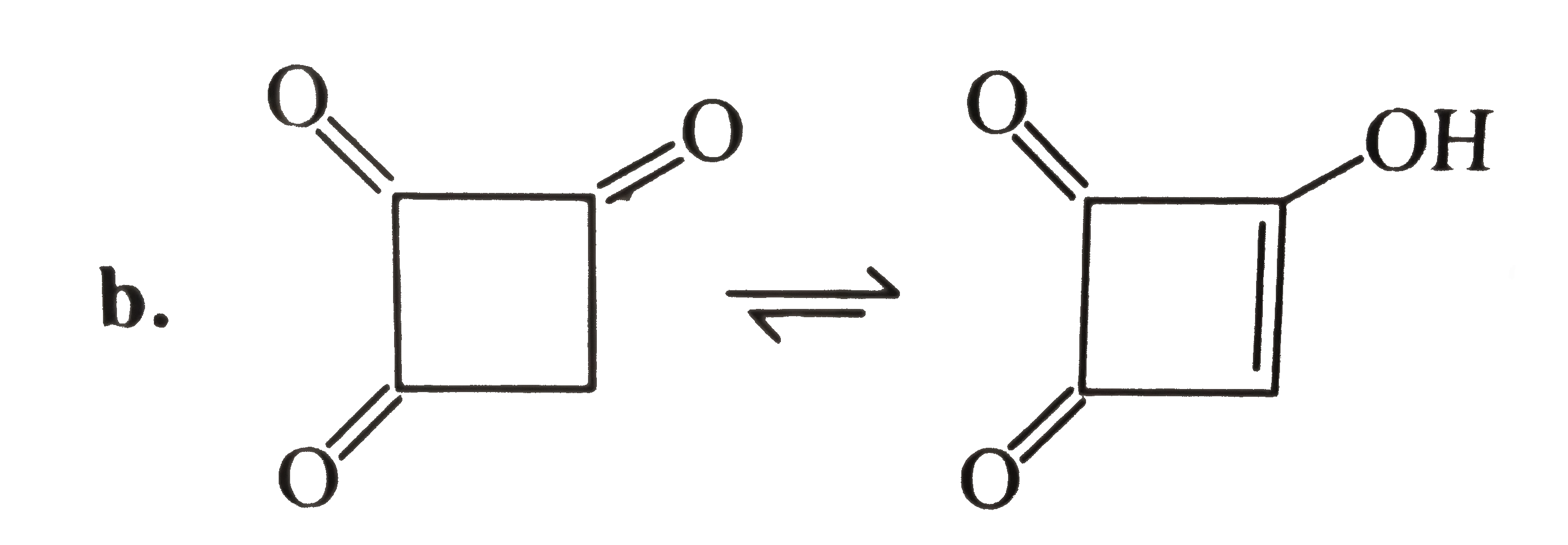
Enolises because its enol has `(4n+2) pi bar e's(i.e., 2pi bar e's, n=0)` and is stabilised by aromaticity
II. It is due to the intermolecular `H-`bonding.
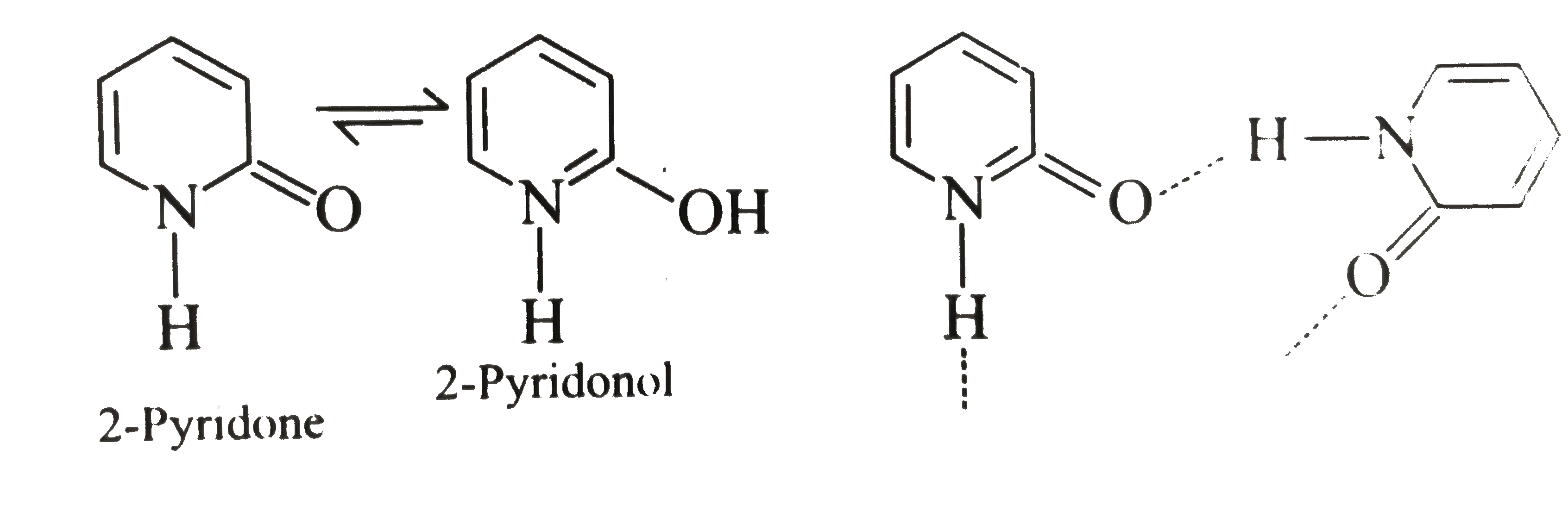
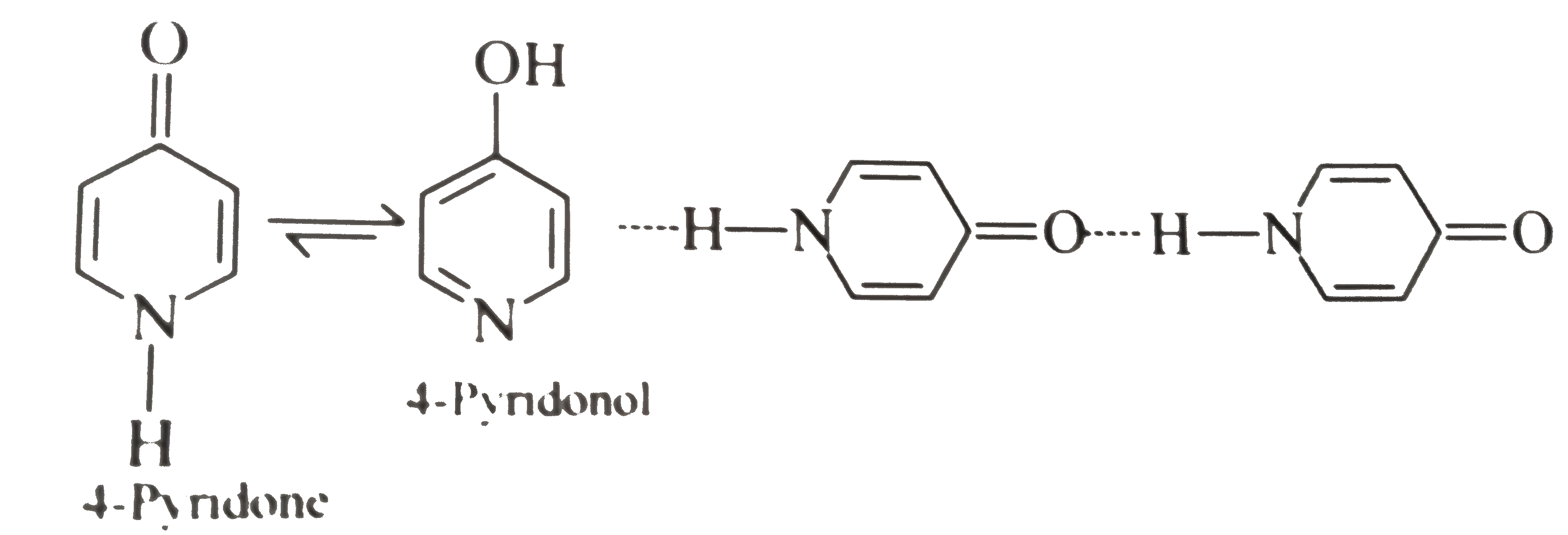
III. The resonance energy of `(C=O)` group is greater than that of the enol.
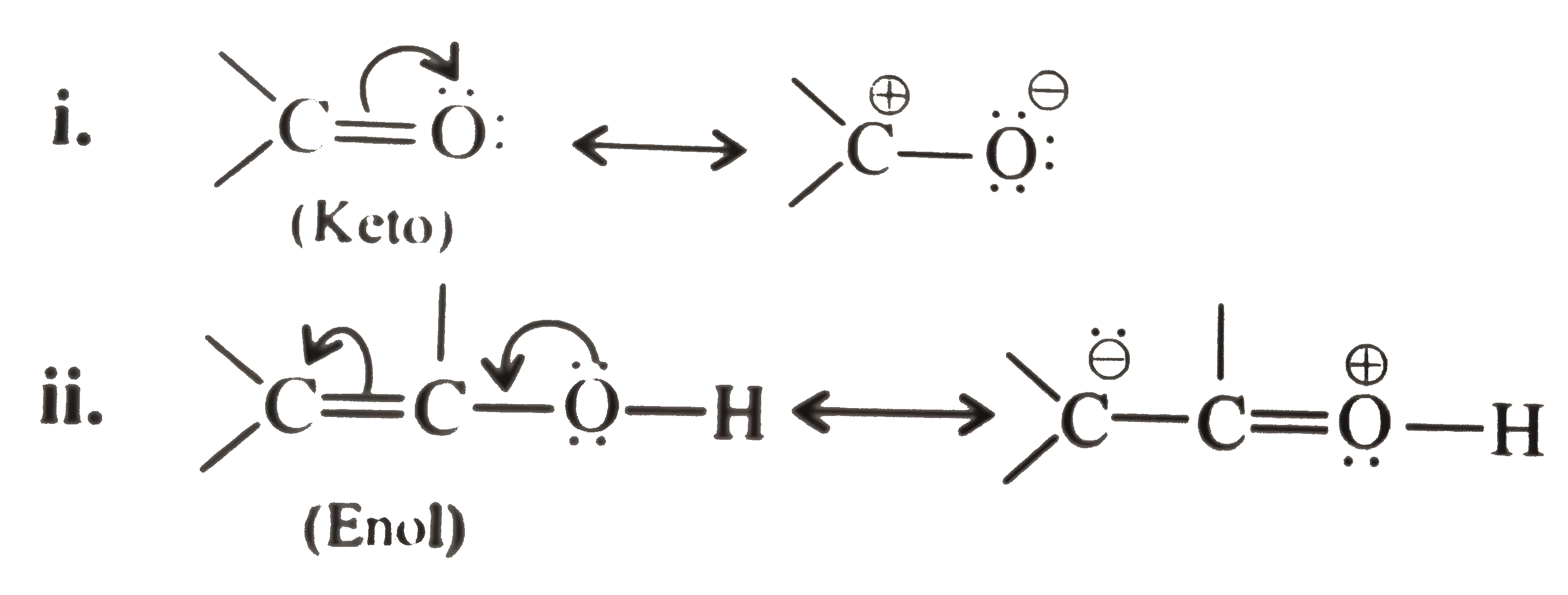
Moreover, `H` atom attached to heteroatoms `(N,O,S, etc)` as in enol form, is more acidic than `(C-H)` bonds as in the keto form, so equilibrium always favours the side with the weaker acid, in this case the keto form.
In keto form, the less `EN`(electronegative) `C` atom is more reactive site but the site with more negative charge (i.e, `O^(Θ)`) is more attracted to the cation present (i.e., `H^(o+)`) and forms enol, thereby lossing the keto reactivity.
IV
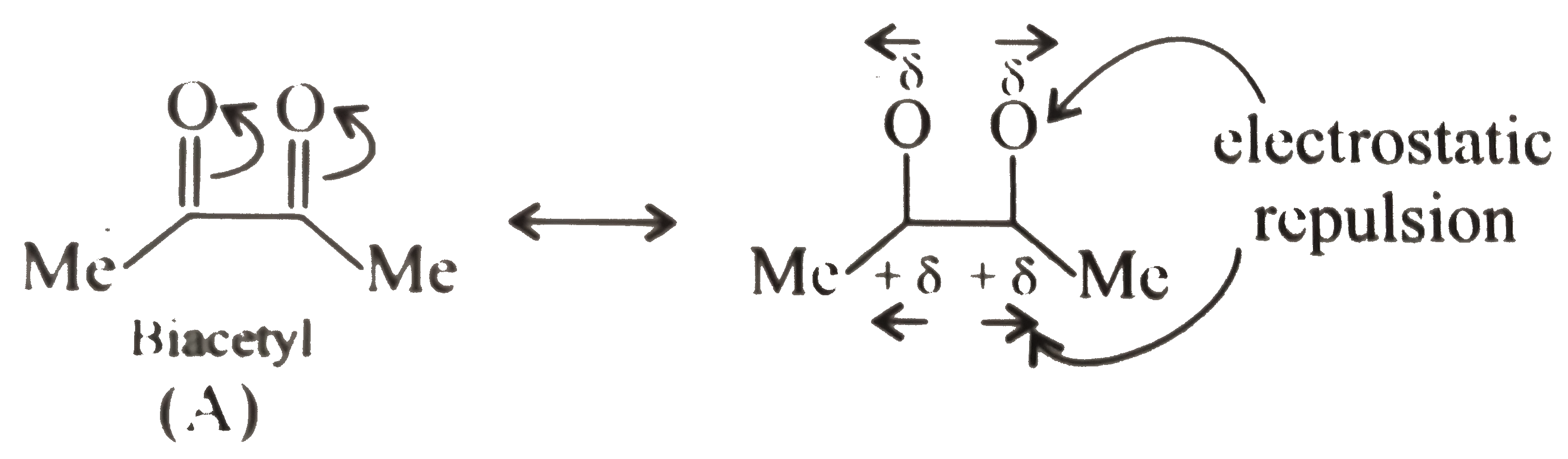
In biacetyl `(A)` adjacent `(C=0)` groups destabilises the molecule due to electrostatic repulsion of the similar charges on the `O` atom of `(C=O)`, but the molecule relieves some of this electrostatic repulsion by acquiring
anti-conformation
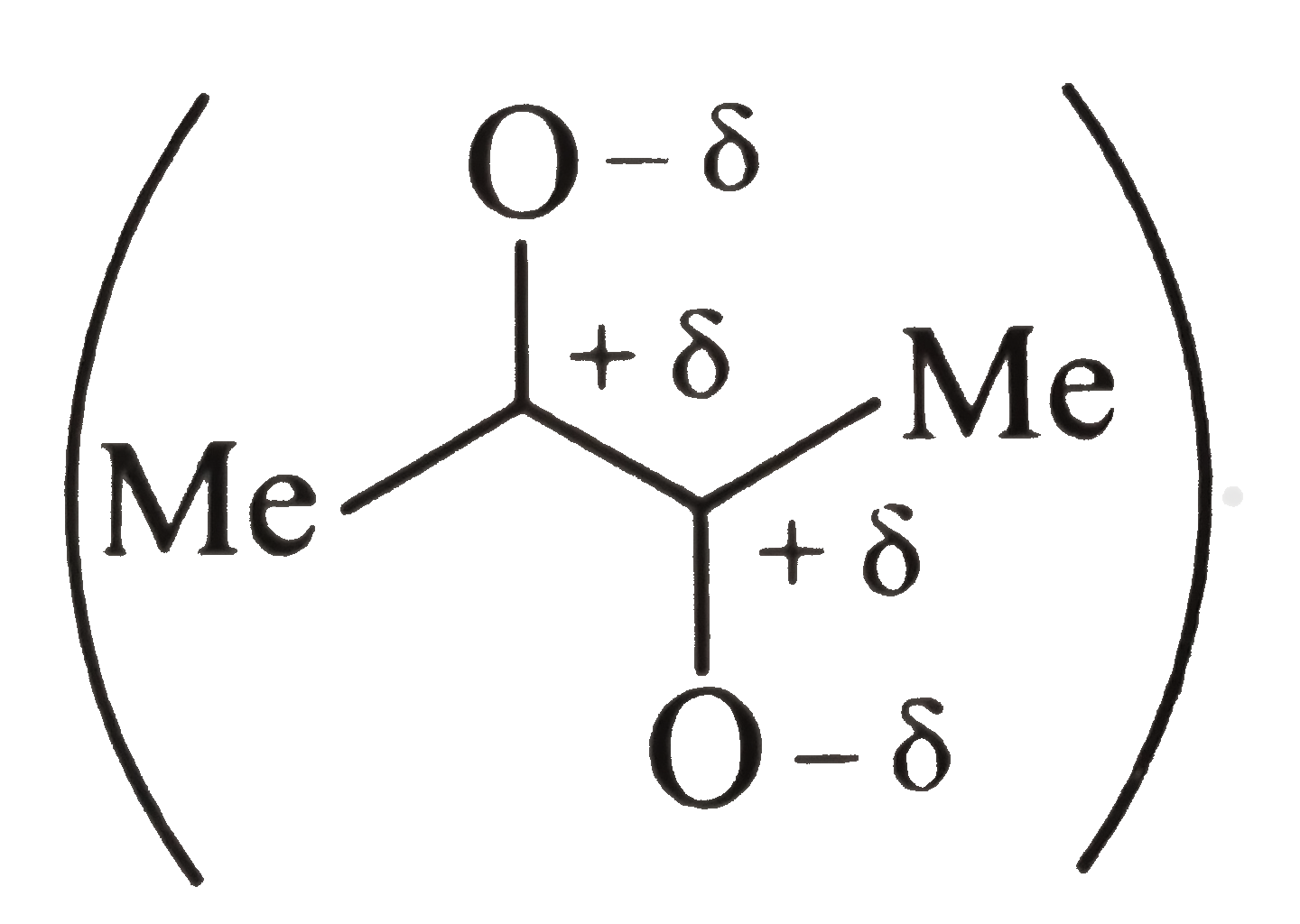
Some repulsion is further relieved in the enol form but enol content is still small because this relief occurs at the expence of loss of `(C=O)` resonance energy.
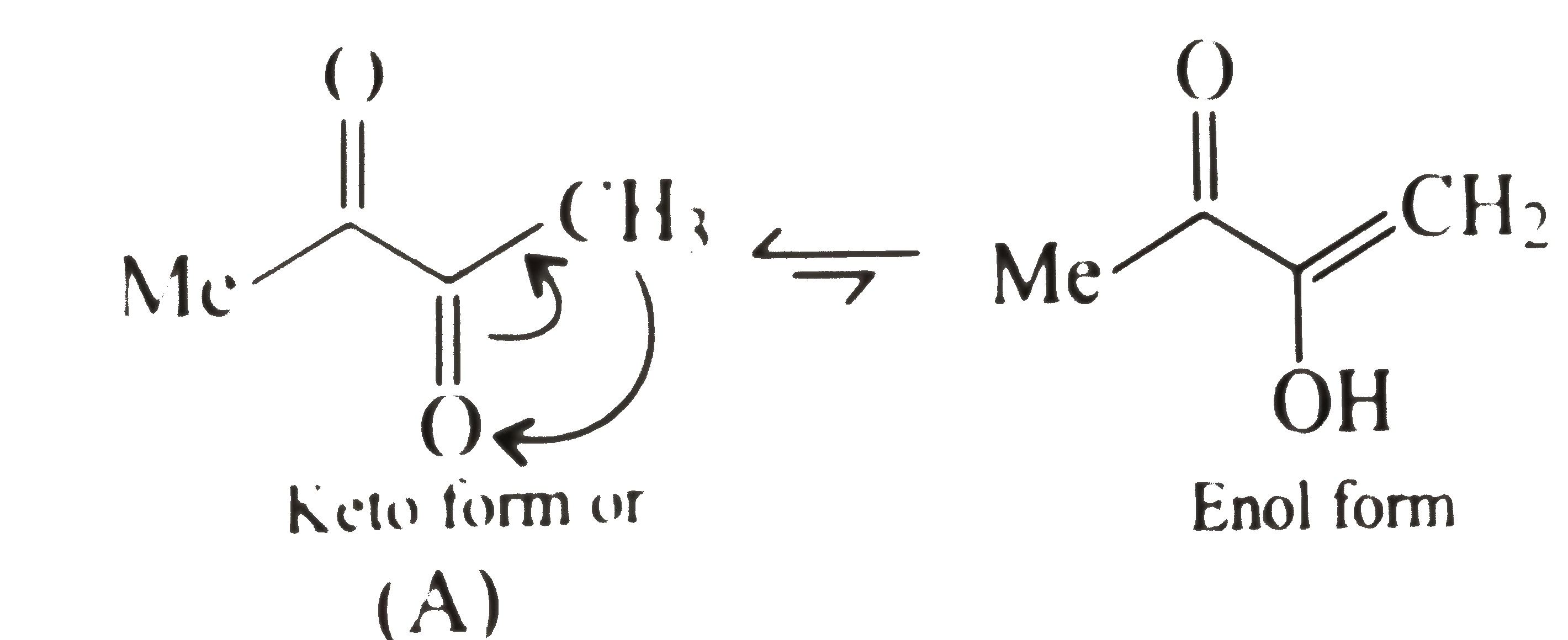
In butan-2-one `(B)`, no other factor except the loss of resonace energy occurs during enolisation.
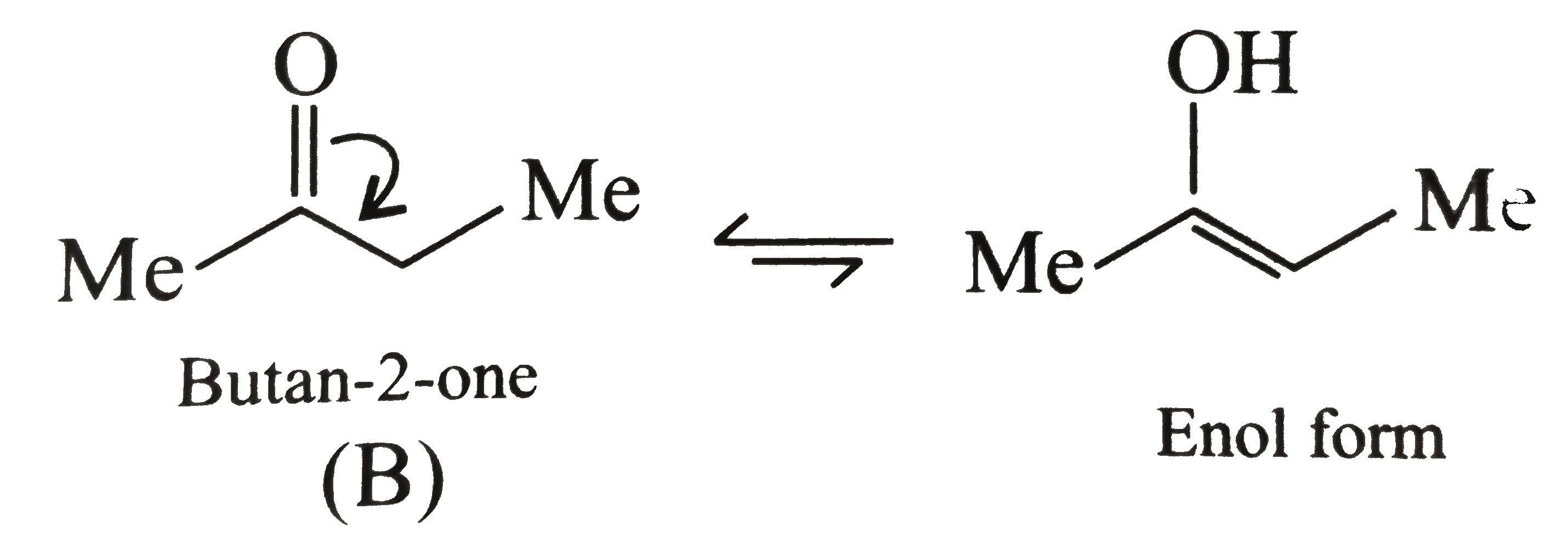
In cyclohexane -`1,2-` dione `(C)`, the cyclohexane rings is rigid and the `-delta` charge on the adjacent `(C=O)` groups is in `syn`-position. Therefore, this molecule relieves its electrostatic repulsion only by the enolisation of one of the `(C=O)` groups
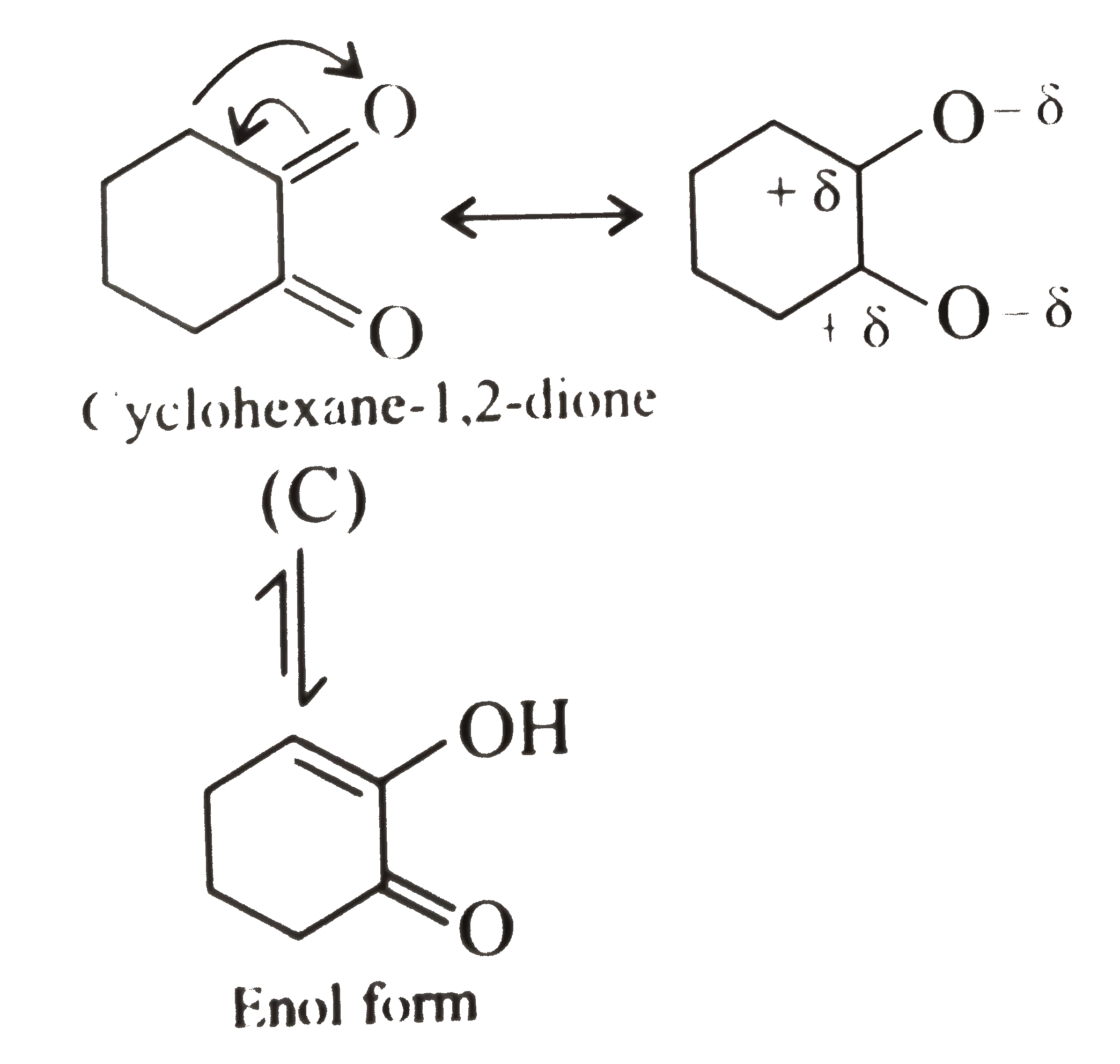
Thus, the enol content of `(A)` is slightly higher than that of `(B)` but much less than `(c )`.