Xenon forms the binary fluorides `XeF_(2),XeF_(4),XeF_(6)` as follows. These are formed by direct combination of Xe and `F_(2)`.
`underset(("Xenon in excess"))(Xe+F_(2))underset("1 bar")overset(673" K")toXeF_(2)`
`underset((1:5" ratio"))(Xe+2F_(2))underset(4" bar")overset(873"K")toXeF_(4)`
`underset((1:20" ratio"))(Xe+3F_(2))underset(60-70" bar")overset(573" K")toXeF_(6)`
Reaction with water :
1) `XeF_(2)` is hydrolysed to form Xe, HF and `O_(2)`
`2XeF_(2)+2H_(2)O to2Xe+4HF+O_(2)`
2) `XeF_(4)` is hydrolysed to give `XeO_(3)`
`6XeF_(4)+12H_(2)O to4Xe+2XeO_(3)+24" HF"+3O_(2)`
3) `XeF_(6)` undergo hydrolysis to form `XeO_(3)`
`XeF_(6)+3H_(2)O to XeO_(3)+6HF`
4) `XeF_(6)` undergo partial hydrolysis to form `XeOF_(4)+XeO_(2)F_(2)`
`XeF_(6)+H_(2)O toXeOF_(4)+2HF`
`XeF_(6)+2H_(2)O to XeO_(2)F_(2)+4HF`
Structure of `XeF_(2)` :
1) In `XeF_(2)` central atom is 'Xe'.
2) 'Xe' undergoes `sp^(3)d` hybridisation in it's `1^(st)` excited state
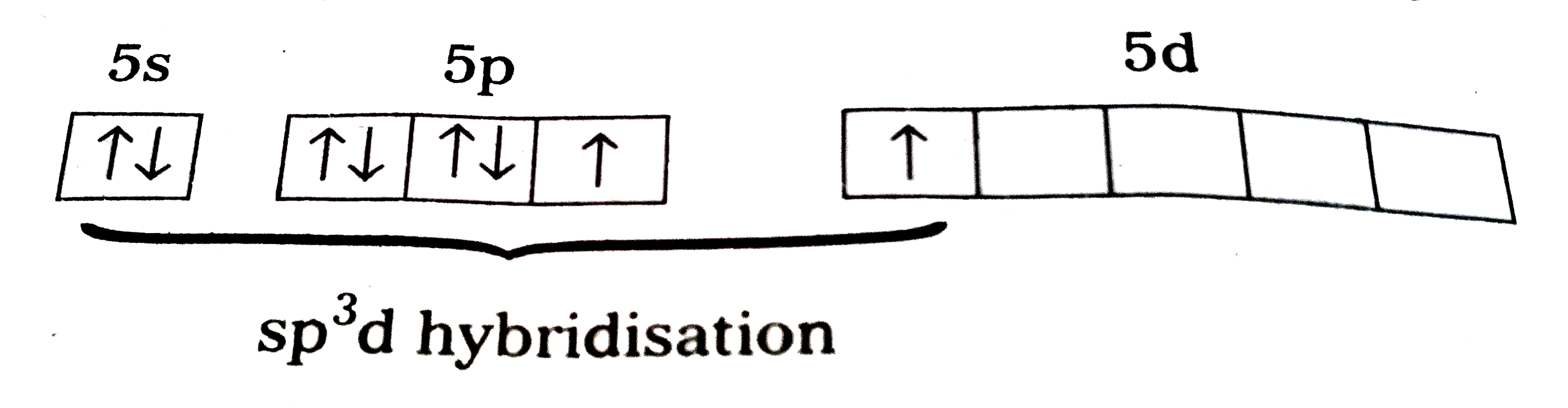
3) Shape of molecule is linear
4) Xe form two `sigma` - bonds with two fluorines.
`"Fe"-underset("xx")overset("xx")(Xe)-"F"`
b) Structure of `XeF_(4)` :
1) Central atom in `XeF_(4)` is 'Xe'.
2) Xe undergoes `sp^(3)d^(2)` hybridisation in it's `2^(nd)` excited. State.

3) Shape of the molecule is square planar with bond angle `90^(@)` and bond length `1.95Å`.
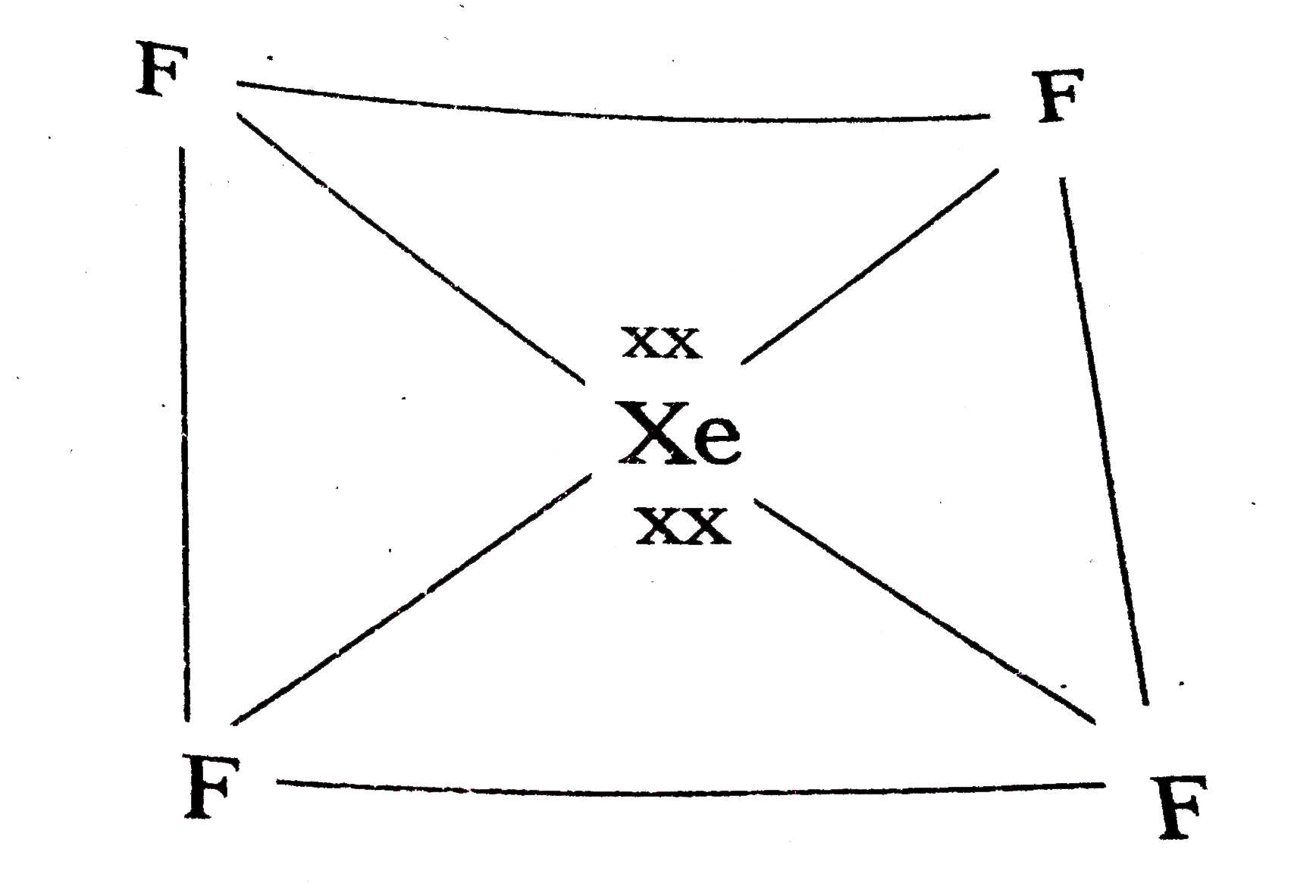
4) Xe - forms `sigma` - bonds by the overlap of `sp^(3)d^(2)-2p_(z)` (F) orbbitals.
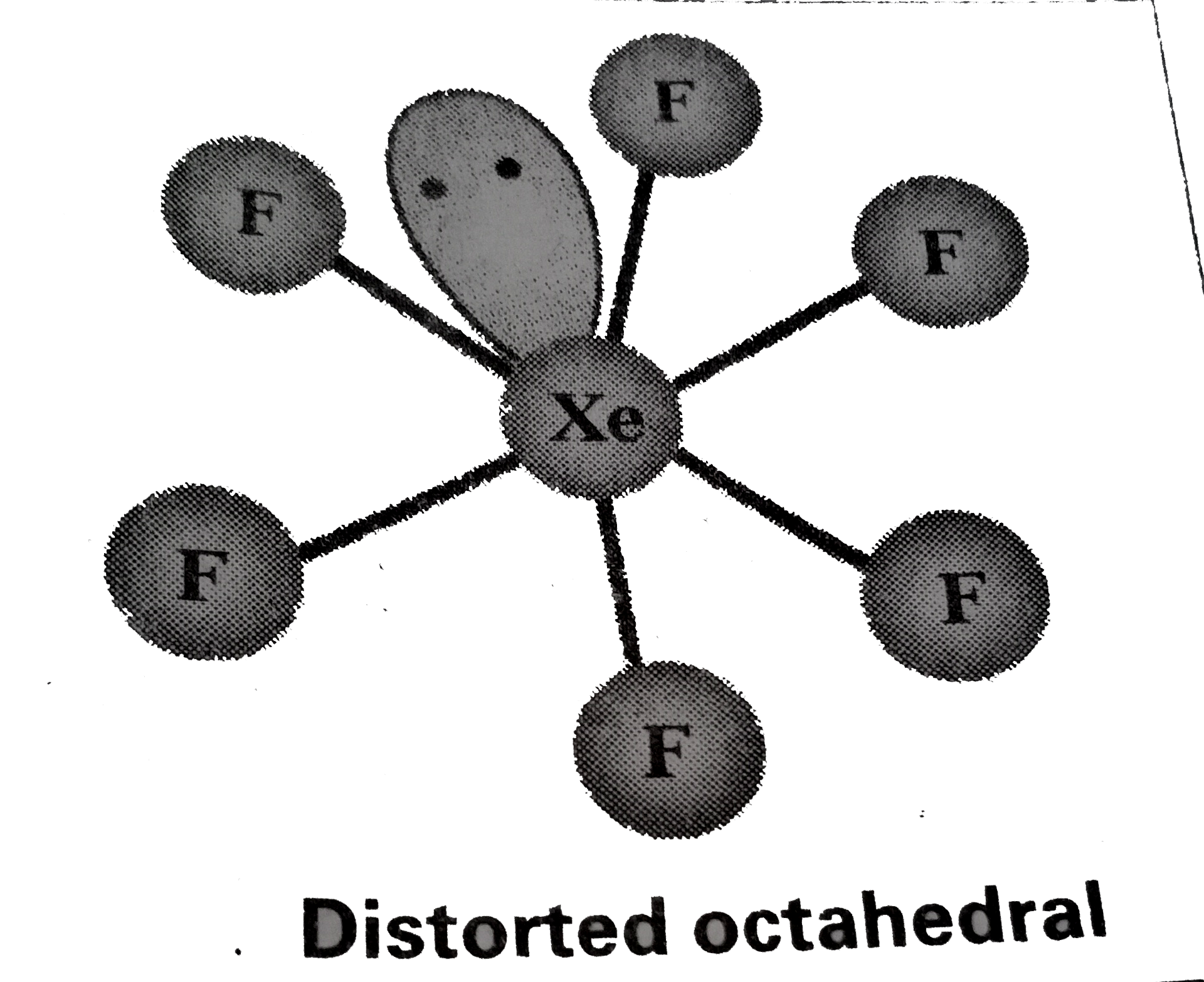
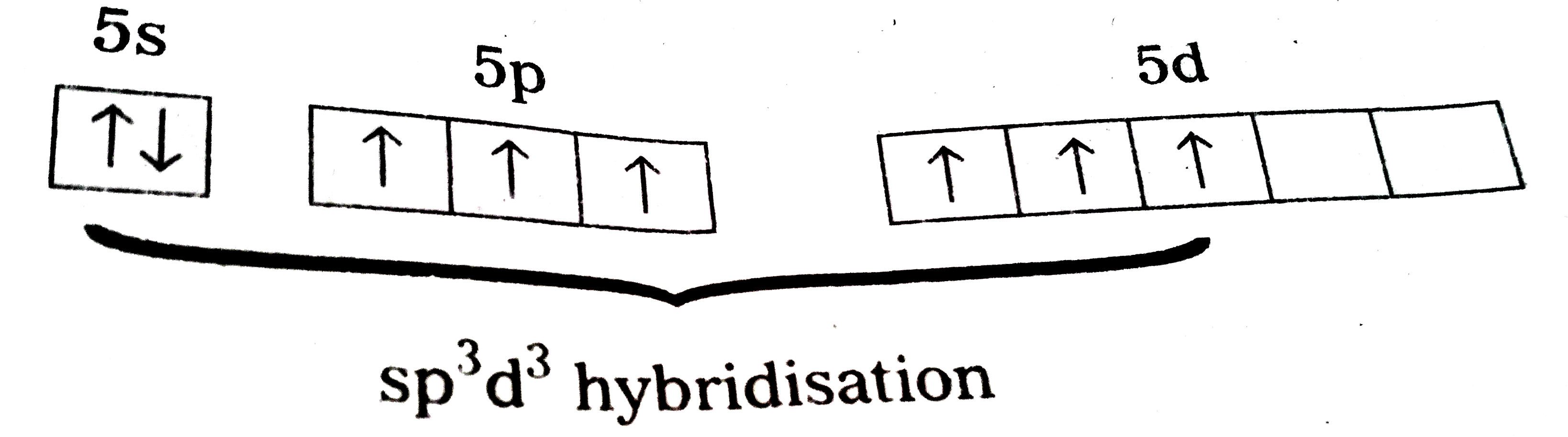
Structure of `XeF_(6)` :
1) Central atom in `XeF_(6)` is 'Xe'
2) Xe under goes `sp^(3)d^(3)` hybbridisation in it's `3^(rd)` excited state.
3) Shape of molecule is distorted octahedral.