Text Solution
Verified by Experts
Topper's Solved these Questions
ORGANIC COMPOUNDS CONTAINING C,H AND O
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise LONG ANSWER QUESTIONS|88 VideosORGANIC COMPOUNDS CONTAINING C,H AND O
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise INTEXT QUESTIONS|54 VideosORGANIC COMPOUNDS CONTAINING C,H AND O
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise VERY SHORT ANSWER QUESTIONS|36 VideosHALOALKANES AND HALOARENES
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise SAQ|9 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise DAM SURE (SAQ - 8 Marks)|15 Videos
Similar Questions
Explore conceptually related problems
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)-ORGANIC COMPOUNDS CONTAINING C,H AND O-SHORT ANSWER QUESTIONS
- Write the products of the reaction.
Text Solution
|
- Write the products of the reaction.
Text Solution
|
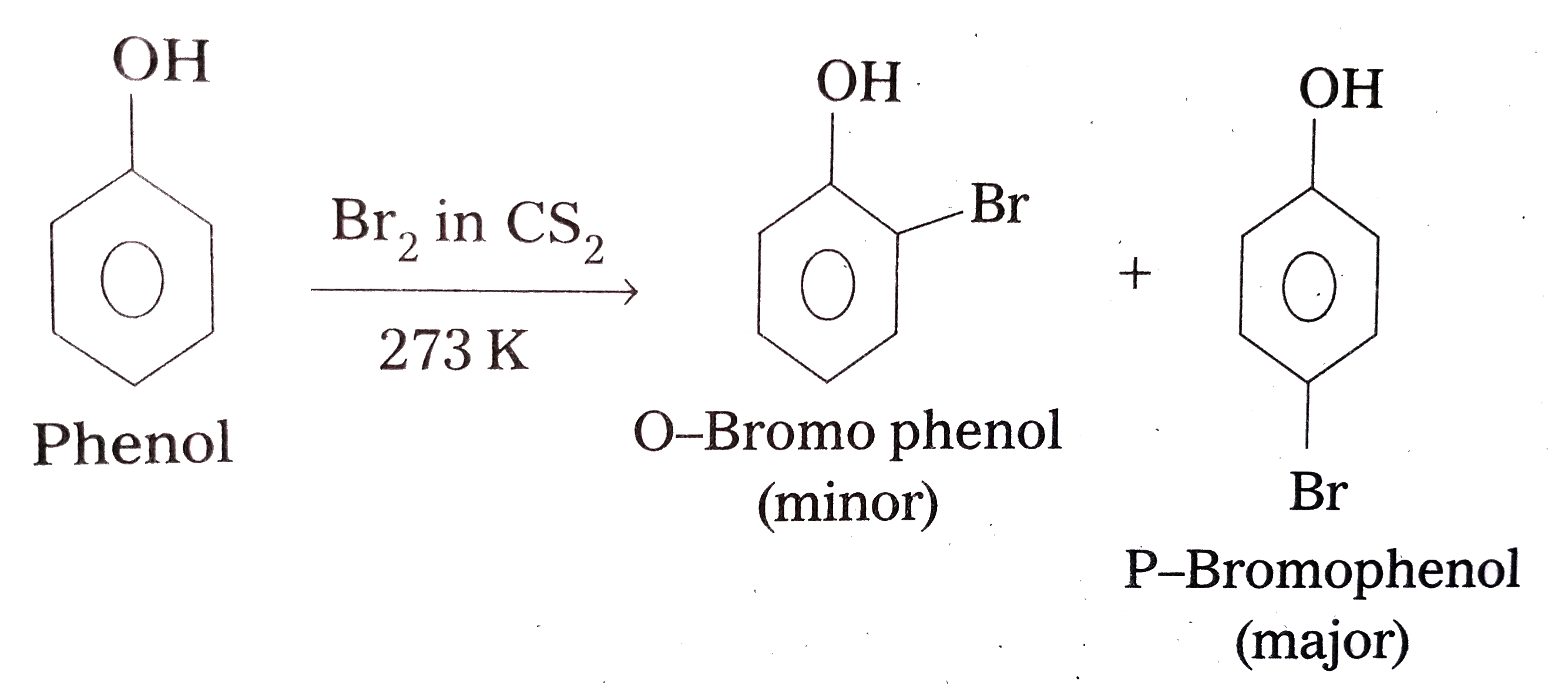
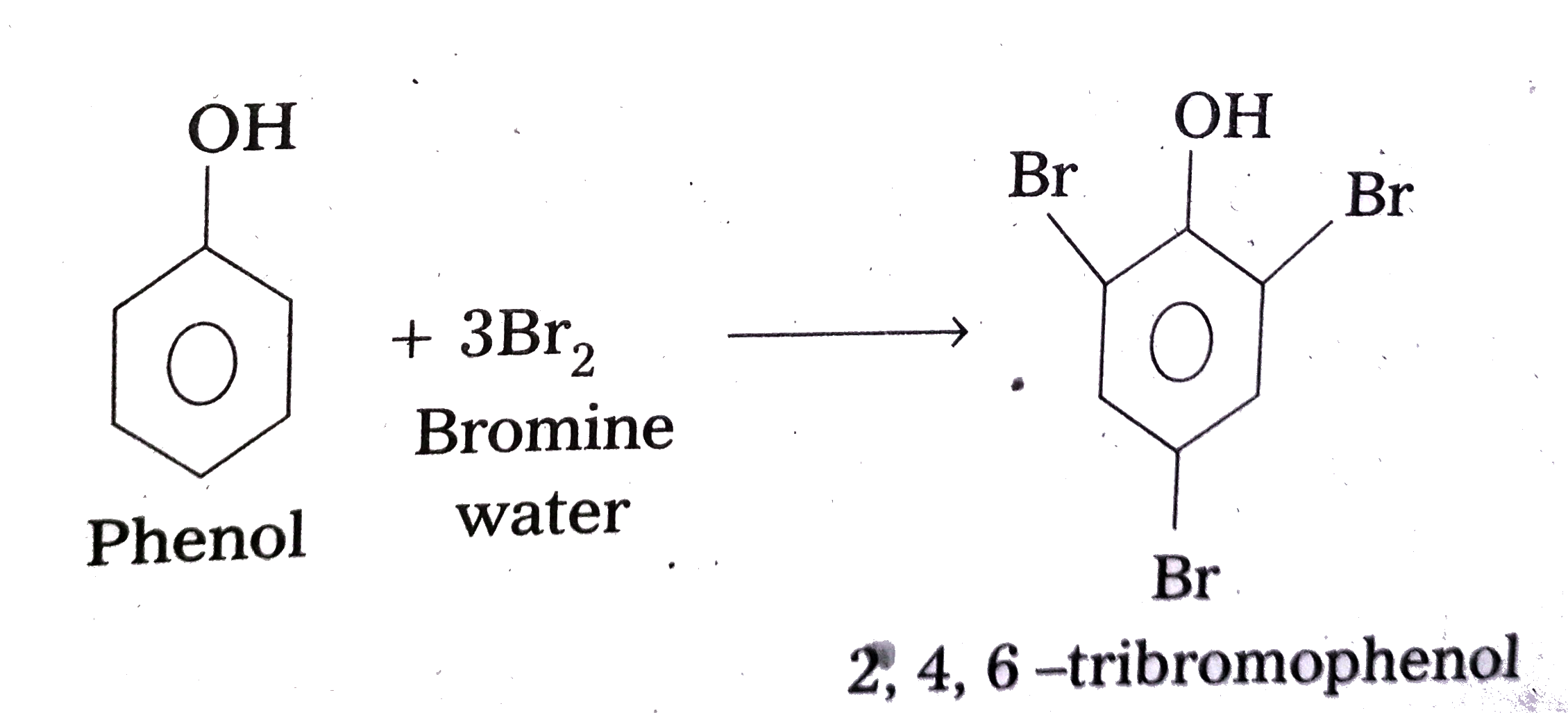
- Explain why phenol with bromine water forms 2,4,6-tribromophenol while...
Text Solution
|
- Explain the acidic nature of phenol.
Text Solution
|
- Explain the electrophilic substitution reaction of Anisole.
Text Solution
|
- Write equations of the Alkylation of anisole reaction.
Text Solution
|
- Write equations of the Nitration of anisole reactions.
Text Solution
|
- Write equation of the Friedel-Crafts acetylation of anisole reaction.
Text Solution
|
- Illustrate hydroboration -oxidation reaction with a suitable example.
Text Solution
|
- Write any two methods for the preparation of phenol.
Text Solution
|
- Write the structure of the 2-Methyl butan -1-ol compound.
Text Solution
|
- Write the structure of the 2,3-diethyl phenol compound.
Text Solution
|
- Write the structure of the 1-ethoxy propane compound.
Text Solution
|
- Write the structure of the Cyclohexyl methanol compound.
Text Solution
|
- Write the equations of any aldehyde with Fehlings reagent.
Text Solution
|
- What is Tollens reagent ? Explain its reaction with Aldehydes.
Text Solution
|
- Write the oxidation products of : Acetaldehyc, Acetone and Acetophenon...
Text Solution
|
- Explain why Aldehydes and ketones undergoes nucleophilic addition whil...
Text Solution
|
- Which the IUPAC name of CH(3)CH(2)CH(Br) CH(2)COOH
Text Solution
|
- Which the IUPAC name of Ph. CH(2)COCH(2)COOH
Text Solution
|