Text Solution
Verified by Experts
Topper's Solved these Questions
ORGANIC COMPOUNDS CONTAINING NITROGEN
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise DAM SURE (SAQ - 8 Marks)|15 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise DAM SURE (VSAQ - 2 Marks)|16 VideosORGANIC COMPOUNDS CONTAINING C,H AND O
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise INTEXT QUESTIONS|54 VideosP-BLOCK ELEMENTS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise INTEXT QUESTIONS|30 Videos
Similar Questions
Explore conceptually related problems
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)-ORGANIC COMPOUNDS CONTAINING NITROGEN-DAM SURE (SAQ - 4 Marks)
- Explain with a suitable example how benzene sulphonylchloride can dist...
Text Solution
|
- How do you prepare Ethyl cyanide and Ethyl isocyanide from a common al...
Text Solution
|
- How do you distinguish cyanides and isocyanides by hydrolysis and redu...
Text Solution
|
- How do you carryout the following conversions ? N - Ethylamine to N,...
Text Solution
|
- How do you carryout the following conversions ? Aniline to Benzene s...
Text Solution
|
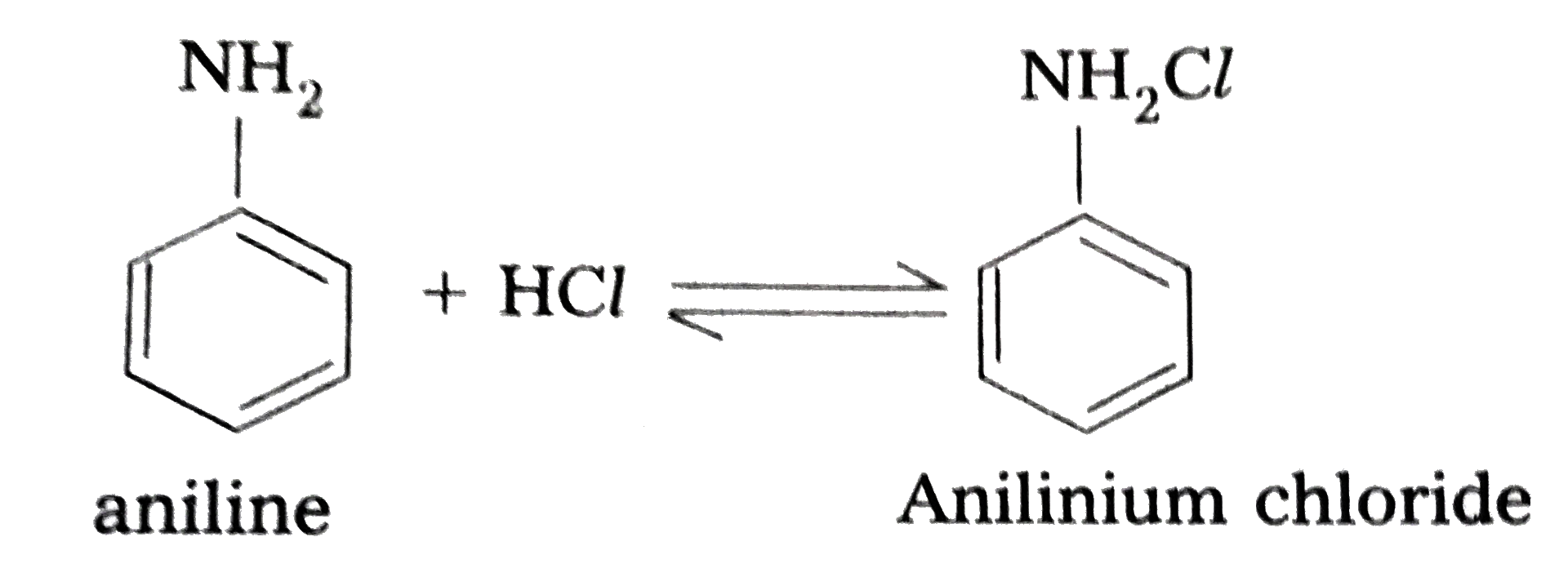
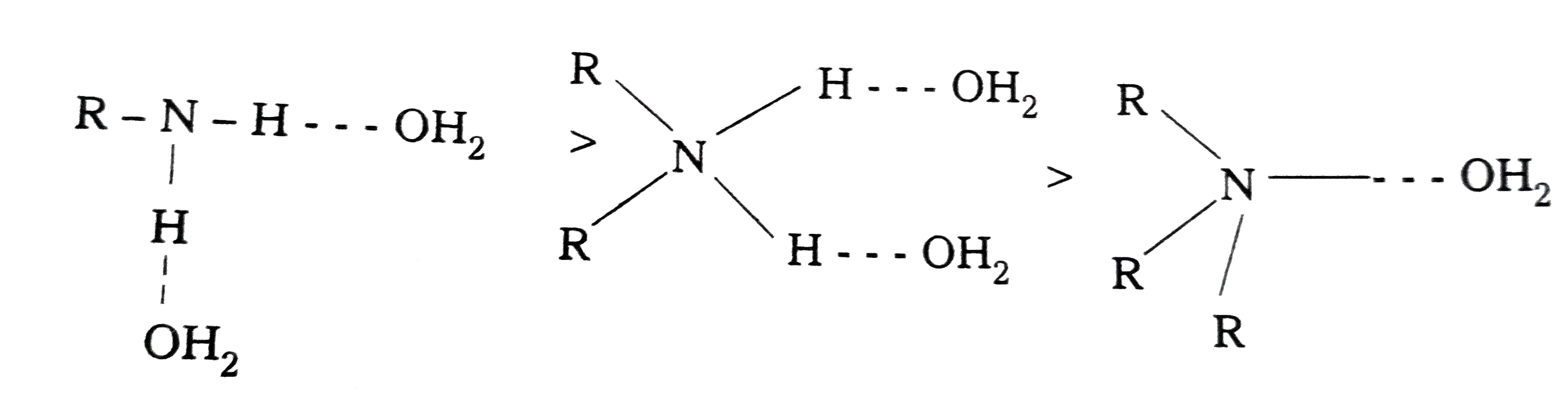
- Explain the basic character of different Amines.
Text Solution
|
- Write two methods each for the preparation of alkyl cyanide and alkyl ...
Text Solution
|
- Give one chemical test of distinguish between the following pairs of c...
Text Solution
|
- Give one chemical test of distinguish between the following pairs of c...
Text Solution
|
- Give one chemical test of distinguish between the following pairs of c...
Text Solution
|