A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
P-BLOCK GROUP 13 - BORON FAMILY
CENGAGE CHEMISTRY|Exercise Exercise (Multiple Correct)|23 VideosP-BLOCK GROUP 13 - BORON FAMILY
CENGAGE CHEMISTRY|Exercise Exercise (Single Correct)|86 VideosP-BLOCK GROUP 13 - BORON FAMILY
CENGAGE CHEMISTRY|Exercise Ex 6.1 (Objective)|14 VideosORGANIC REACTION MECHANISM
CENGAGE CHEMISTRY|Exercise Analytical and Descriptive|6 VideosP-BLOCK GROUP 14 - CARBON FAMILY
CENGAGE CHEMISTRY|Exercise Exercises Archives (Subjective)|9 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-P-BLOCK GROUP 13 - BORON FAMILY-Exercise (Linked Comprehension)
- "Colemanite" + Na(2)CO(3) overset("Fused")rarr (A) + (B) + CO(2) und...
Text Solution
|
- "Colemanite" + Na(2)CO(3) overset("Fused")rarr (A) + (B) + CO(2) und...
Text Solution
|
- Boron with hydrogen forms a number of hydrides which are known are bor...
Text Solution
|
- Boron with hydrogen forms a number of hydrides which are known are bor...
Text Solution
|
- Boron with hydrogen forms a number of hydrides which are known are bor...
Text Solution
|
- Boron with hydrogen forms a number of hydrides which are known are bor...
Text Solution
|
- Boron with hydrogen forms a number of hydrides which are known are bor...
Text Solution
|
- Boron with hydrogen forms a number of hydrides which are known are bor...
Text Solution
|
- Boron with hydrogen forms a number of hydrides which are known are bor...
Text Solution
|
- Boron reacts with oxygen at 700^@C to give (A). Compound (A) reacts wi...
Text Solution
|
- Boron reacts with oxygen at 700^@C to give (A). Compound (A) reacts wi...
Text Solution
|
- Boron reacts with oxygen at 700^@C to give (A). Compound (A) reacts wi...
Text Solution
|
- Boron reacts with oxygen at 700^@C to give (A). Compound (A) reacts wi...
Text Solution
|
- Boron reacts with oxygen at 700^@C to give (A). Compound (A) reacts wi...
Text Solution
|
- Boron reacts with oxygen at 700^@C to give (A). Compound (A) reacts wi...
Text Solution
|
- Boron reacts with oxygen at 700^@C to give (A). Compound (A) reacts wi...
Text Solution
|
- Borax is actually made of two tetrahedra and two triangular units join...
Text Solution
|
- Borax is actually made of two tetrahedra and two triangular units join...
Text Solution
|
- The molecular shapes of diborane is shown below : . Consider the f...
Text Solution
|
- The molecular shapes of diborane is shown below : . Select correct...
Text Solution
|
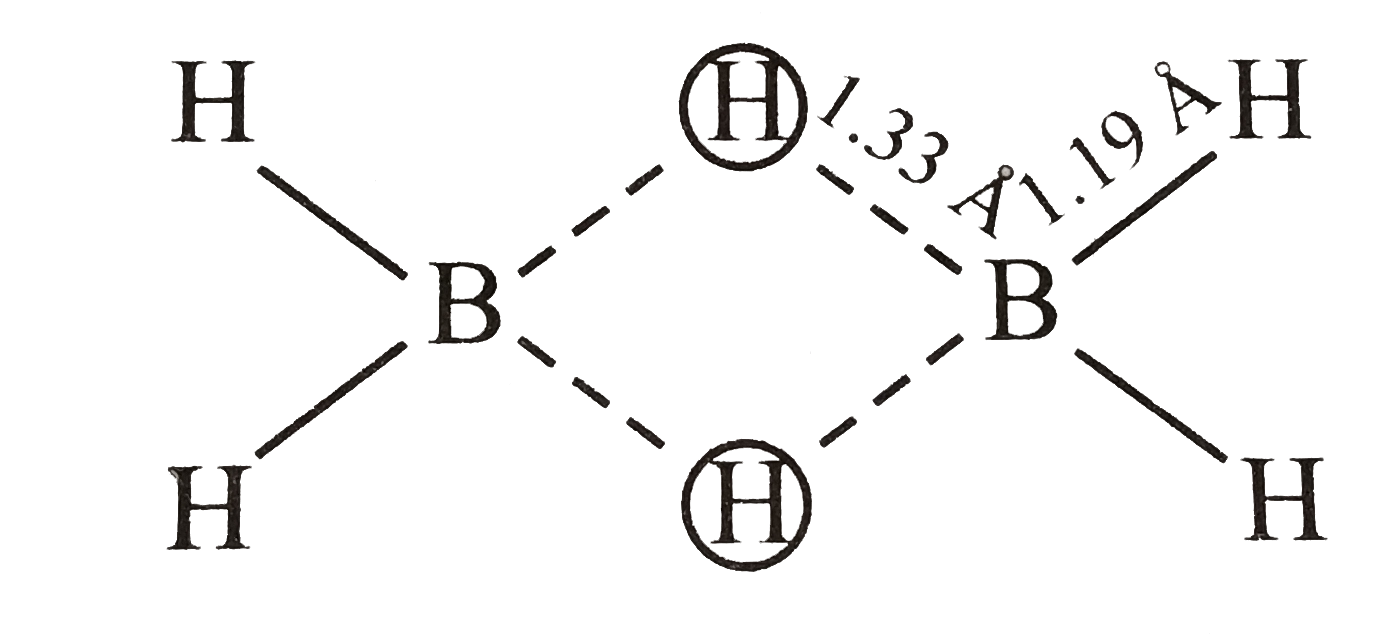 .
.