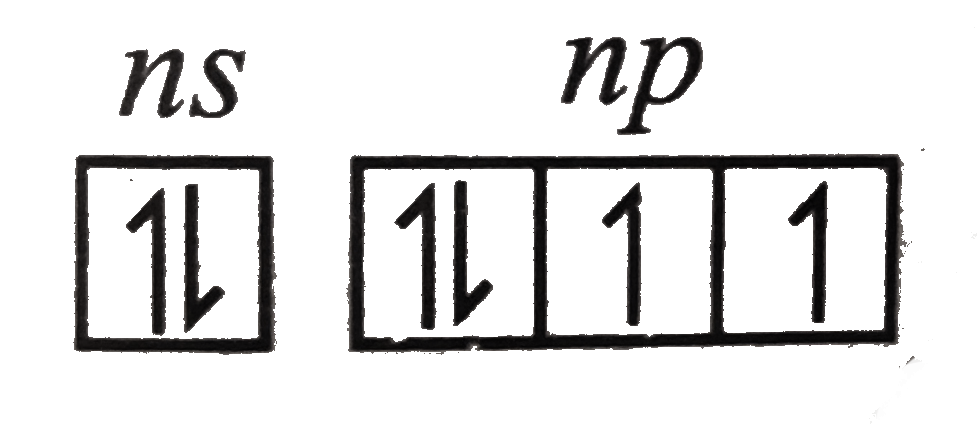Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
P-BLOCK GROUP 16 ELEMENTS - THE OXYGEN FAMILY
CENGAGE CHEMISTRY|Exercise Exercises Fill In The Blanks|30 VideosP-BLOCK GROUP 16 ELEMENTS - THE OXYGEN FAMILY
CENGAGE CHEMISTRY|Exercise Exercises True/False|15 VideosP-BLOCK GROUP 16 ELEMENTS - THE OXYGEN FAMILY
CENGAGE CHEMISTRY|Exercise Exercises Single Correct|77 VideosP-BLOCK GROUP 15 ELEMENTS - THE NITROGEN FAMILY
CENGAGE CHEMISTRY|Exercise Exercises Archives (Subjective)|28 VideosP-BLOCK GROUP 17 ELEMENTS - THE HALOGEN FAMILY
CENGAGE CHEMISTRY|Exercise True/False (Subjective)|14 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-P-BLOCK GROUP 16 ELEMENTS - THE OXYGEN FAMILY-Exercises Integer
- The number of unpaired electrons in the valence shell of the members o...
Text Solution
|
- What is oxidation state of sulphur is Caro's acid ?
Text Solution
|
- How many orbitals are involved in the hybridisation of sulphur in SCl2...
Text Solution
|
- How many pi-bonds are present in Marshall's acid ?
Text Solution
|
- Ozone reacts with dry iodine to form an oxide having oxygen atoms in i...
Text Solution
|
- How many lone pairs are present in OF2 molecule ?
Text Solution
|
- What is the atomicity of S in sulphur in sulphate ion ?
Text Solution
|
- What is the oxidation state of sulphur in sulphate ion ?
Text Solution
|
- How many S-S bonds are present in S8 molecule ?
Text Solution
|
- Among the oxides gives below, how many are acidic ? CrO3, Mn2 O7, CO, ...
Text Solution
|
- In how many of the following species, S-atom is sp^3 hybridised ? S8...
Text Solution
|
- What is the number of sigma bonds present in peroxodisulphuric acid ?
Text Solution
|
- What is the bond order of O2 molecule ?
Text Solution
|
- Conc. H2 SO4 reacts with four moles of Ag to give moles of Ag2 SO4.
Text Solution
|