(a) (i) is most stable because it has no formal charges.
(ii) (III) is least stable because it has an `overline e-`deficient carbon. In (III), `C1` uses an empty `3d` orbital to accommodate a pair of `overline e^, s` which is not possible in the `C` atom. Thus, the order of stability is `(I) gt (II) gt (III)`
(b) (i) `(IV)` and `(V)` have greater number of convalent bonds , hence, these are more stable than either `(VI)` or `(VII)` . Furthermore between `(IV)` and `(V),(IV)` has no formal charges and hence is more stable than the `(V)`
(ii) `(VII)` is less stable than `(VI)` because in `(VII)` the positive charge is on oxygen, which is a more `EN` atom than carbon on which positive charge is present in `(VI)`. Thus, the order of stability is `(IV) gt (V) gt (VI) gt (VII)`
( c) In the resonating structure of phenol as shown below, structure `(I)` and `(II)` are more stable than the others.
Structures `(III),(IV)`, and `(V)` are less stable due to charge separation and positive charge on oxygen atom.
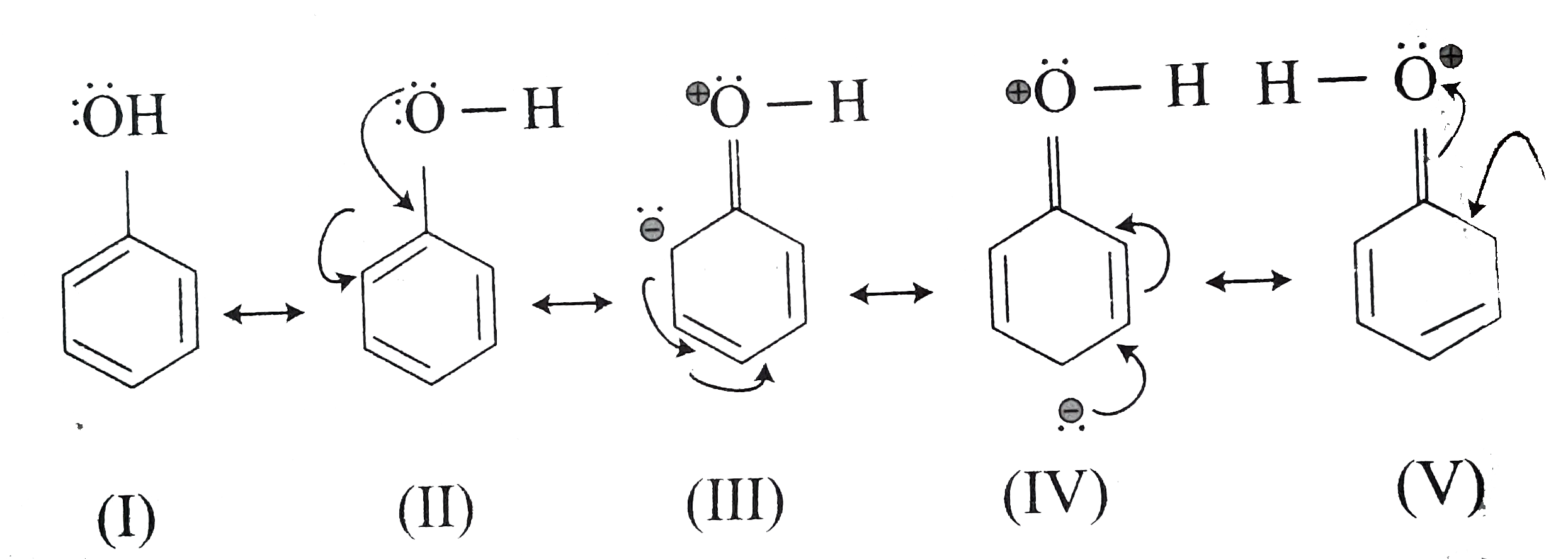
Order of stability `rArr (I)=(II)gt(III)=(V) gt (IV)`
(I)and (II) `rArr` Unchanged structures
`(IV) rArr` More charge separation
Order of energy `rArr (IV) gt (III)=(V)gt (I)=(II)`
(III)and (V) rArr Less charge separation.
