Text Solution
Verified by Experts
Topper's Solved these Questions
ALCOHOL,PHENOL AND ETHERS
CENGAGE CHEMISTRY|Exercise Exercises Linked Comprehension|60 VideosALCOHOL,PHENOL AND ETHERS
CENGAGE CHEMISTRY|Exercise Exercises Multiple Correct|35 VideosALCOHOL,PHENOL AND ETHERS
CENGAGE CHEMISTRY|Exercise Exercises Subjective|25 VideosALIPHATIC AND AROMATIC ALDEHYDES AND KETONES
CENGAGE CHEMISTRY|Exercise Archives Subjective|29 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-ALCOHOL,PHENOL AND ETHERS-Exercises Concept Application
- Give two reactions that show the acidic nature of phennol. Compare the...
Text Solution
|
- Explain why is ortho-nitrophenol more acidic than ortho-methoxyphenol ...
Text Solution
|
- Explain how does the (---OH) group attached to a carbon of benzene rin...
Text Solution
|
- Give the equations of the following reactions: i. Oxidation of prop...
Text Solution
|
- Explain the following with an example: i. Kolbe's reaction ii. R...
Text Solution
|
- Write the mechanism of hydration of ethene to yield ethanol.
Text Solution
|
- How are the following conversions carried out ? i. Propene rarr Pro...
Text Solution
|
- Name the reagents used in the following reactions: i. Oxidation of ...
Text Solution
|
- Given reason for the higher boiling point of ethanol in comparison to ...
Text Solution
|
- Give the IUPAC names of the following ethers: C(2)H(5)OCH(2)--overse...
Text Solution
|
- Write the names of reagents and equations for the preparation of follo...
Text Solution
|
- Illustrate with examples the limitations of Williamson's synthesis for...
Text Solution
|
- How is 1-propoxyproapane synthesised from propan-1-ol ? Write mechanis...
Text Solution
|
- Preparation of ethers by acid dehydration of secondary or tetiary alco...
Text Solution
|
- Write the equation of the reaction of hydrogen iodide with : (i) 1-p...
Text Solution
|
- Explain the fact that in aryl ethers, (i) the alkoxy group activates t...
Text Solution
|
- Write the mechanism of the reaction of Hiwith methoxymethane.
Text Solution
|
- Write the equations of the following reactions: i. Friedel-Crafts r...
Text Solution
|
- Show how would you synthesisse the following alcohols form approprite ...
Text Solution
|
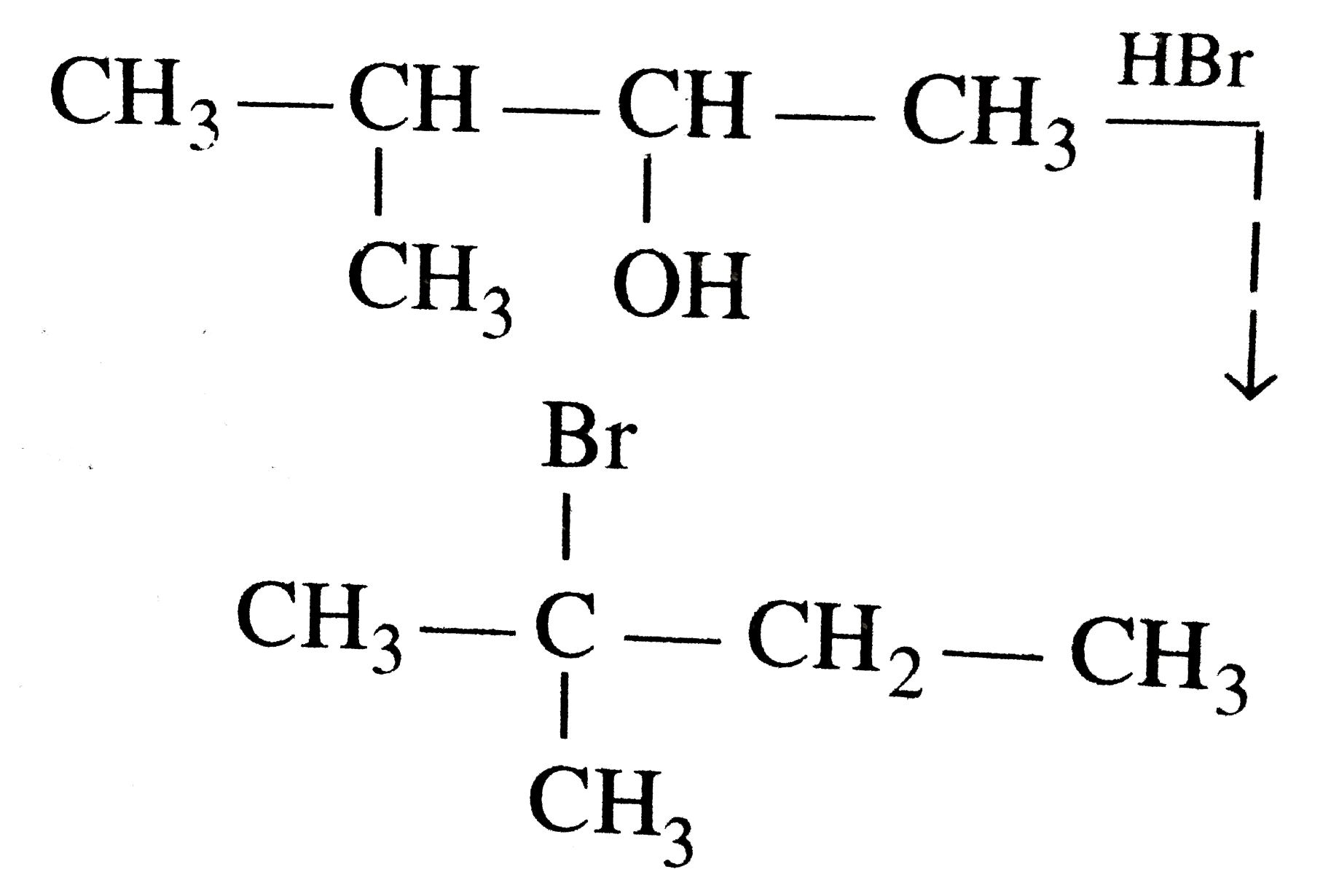
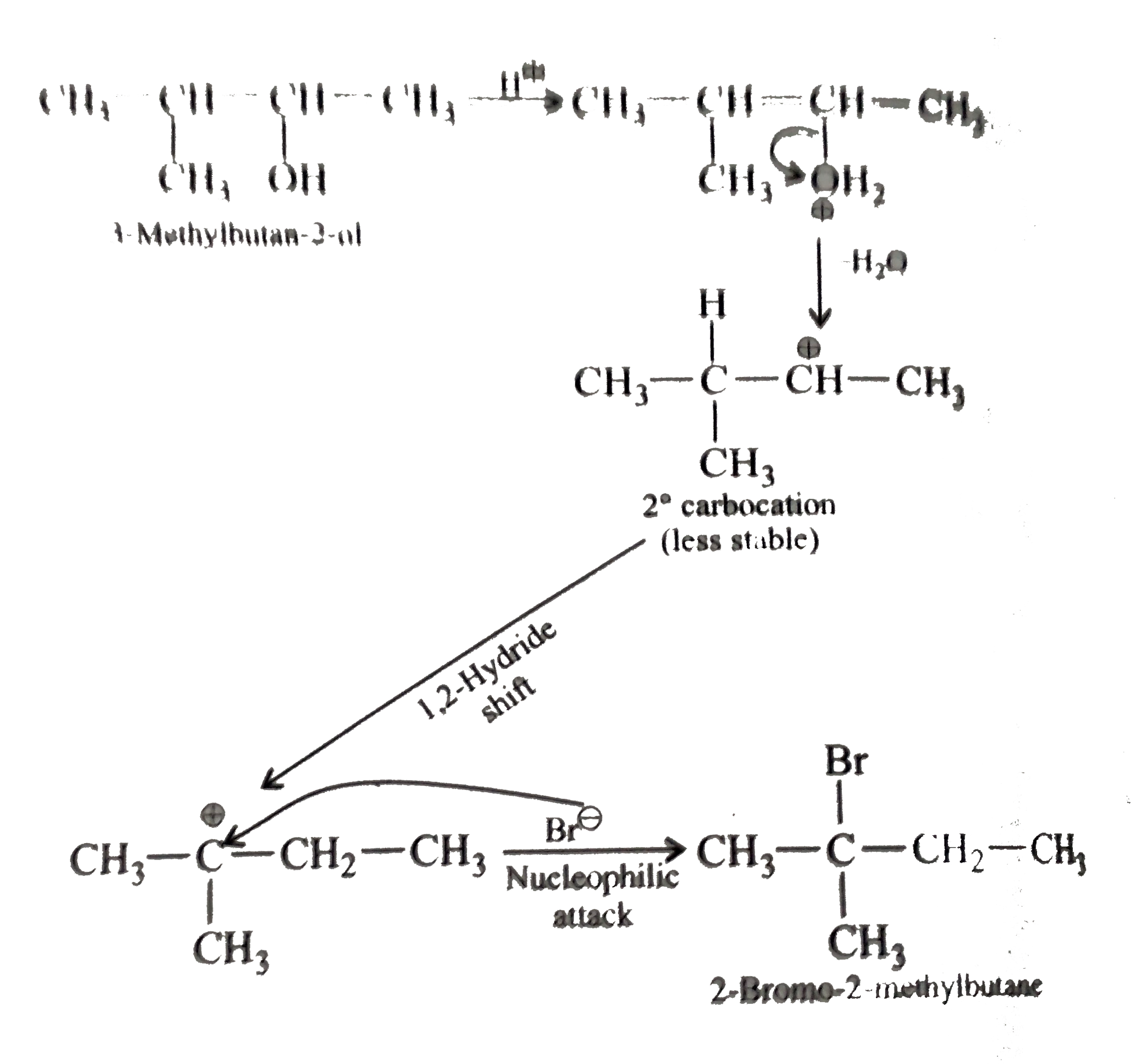
- when 3-methylbutan-2-ol is treated with HBr, the following reaction ta...
Text Solution
|