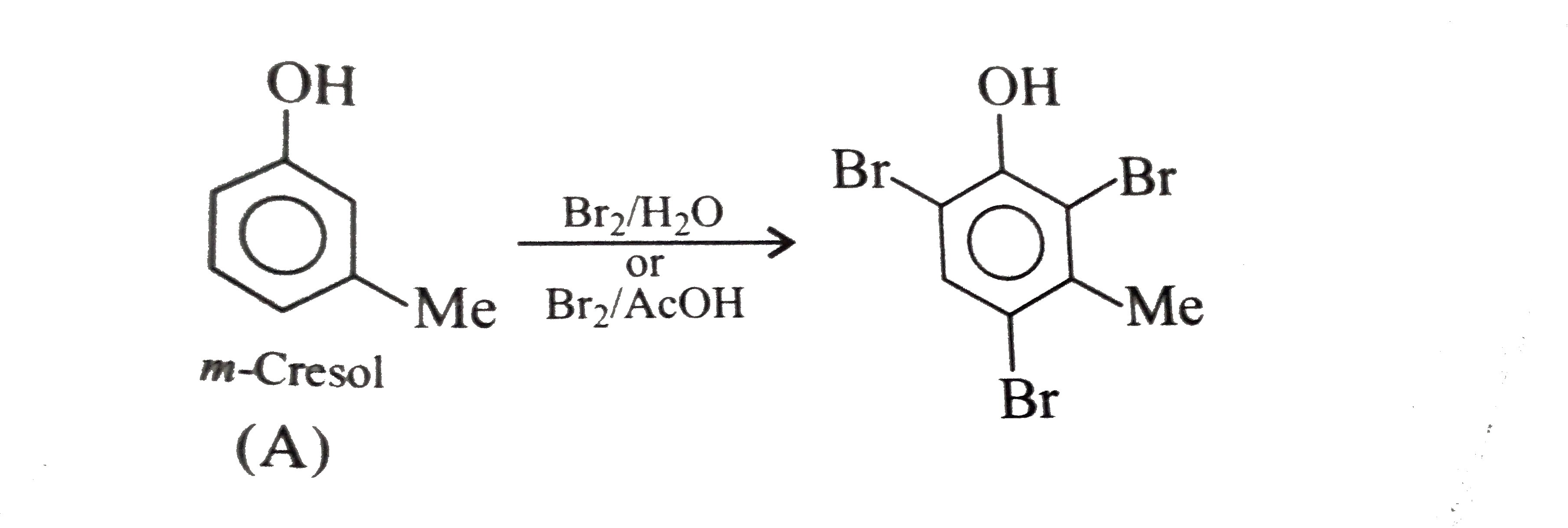
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ALCOHOL,PHENOL AND ETHERS
CENGAGE CHEMISTRY|Exercise Exercises Assertion-Reasoning|10 VideosALCOHOL,PHENOL AND ETHERS
CENGAGE CHEMISTRY|Exercise Archives Single Correct|14 VideosALCOHOL,PHENOL AND ETHERS
CENGAGE CHEMISTRY|Exercise Exercises Multiple Correct|35 VideosALIPHATIC AND AROMATIC ALDEHYDES AND KETONES
CENGAGE CHEMISTRY|Exercise Archives Subjective|29 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-ALCOHOL,PHENOL AND ETHERS-Exercises Single Correct
- Which of the following alcohols has the highest boiling point ?
Text Solution
|
- Grain alcohol, a product of yeast fermentaion of sagars in grains and ...
Text Solution
|
- Which of the following is wood alcohol ?
Text Solution
|
- Rubbing alcohol, used rubdowns, on its evaporation reduces fever. It i...
Text Solution
|
- Fusel oil (Greek, inferior oil) is a mixture of alcohols otained in sm...
Text Solution
|
- Proof is defined as ….. the percentage by volume of alcohol in ethanol...
Text Solution
|
- Wine with 24 proof is …… by volume of alcohol.
Text Solution
|
- Which of the following is most basic in character ?
Text Solution
|
- The intestinal antiseptic Salol is correctly represented as:
Text Solution
|
- Which of the following is produced during the following reaction ? C...
Text Solution
|
- Which among the following compounds will not give effervescence with s...
Text Solution
|
- Which of the following will react faster with Lucas reagent ?
Text Solution
|
- The compound that will react with HBr most readily is:
Text Solution
|
- Amongst the following phenols which one is most acidic ?
Text Solution
|
- An organic compound (A) with molecular formula C(7)H(8)O dissolves in ...
Text Solution
|
- Identify Z in the following seriesgt C(2)H(5)OH overset(PBr(3))rarr X...
Text Solution
|
- The strongest acid among the following aromatic compound is:
Text Solution
|
- A compound (A) has a molecular formula C(2)CI(3)OH. It reduces Fehling...
Text Solution
|
- Brandy is distilled form fermented fruits, rum is distilled form molas...
Text Solution
|
- The compound (A) is:
Text Solution
|