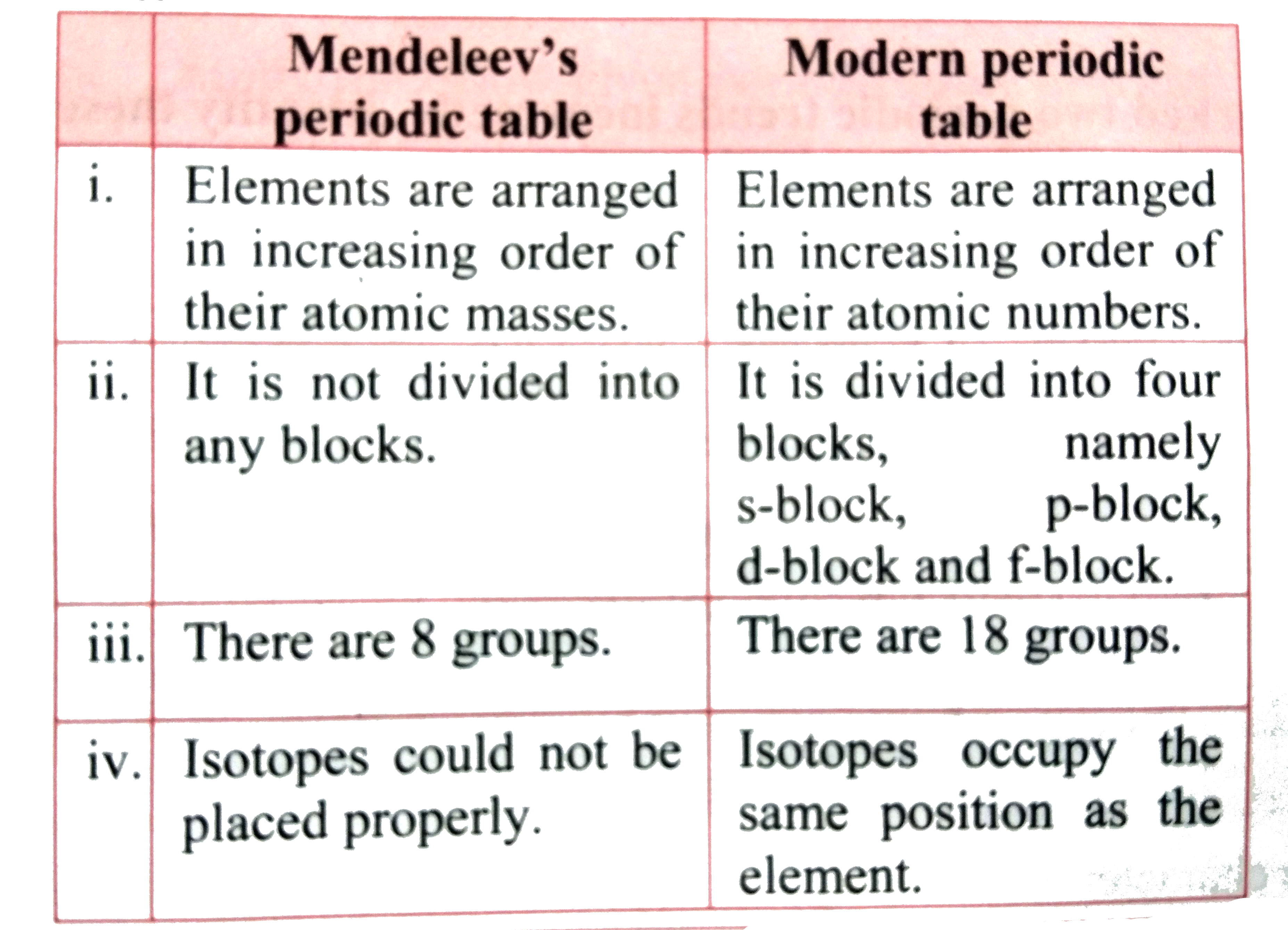
Text Solution
Verified by Experts
Topper's Solved these Questions
PERIODIC CLASSIFICATION OF ELEMENTS
TARGET PUBLICATION|Exercise Complete the given chart/table|2 VideosPERIODIC CLASSIFICATION OF ELEMENTS
TARGET PUBLICATION|Exercise Questions based on diagram|16 VideosPERIODIC CLASSIFICATION OF ELEMENTS
TARGET PUBLICATION|Exercise Give reasons|5 VideosMETALLURGY
TARGET PUBLICATION|Exercise CHAPTER ASSESSMENT|19 Videos
Similar Questions
Explore conceptually related problems