Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
TARGET PUBLICATION-PERIODIC CLASSIFICATION OF ELEMENTS -Apply your knowledge
- Is the number of valence electrons same for all these elements?
Text Solution
|
- Is the number of shells the same in these ?
Text Solution
|
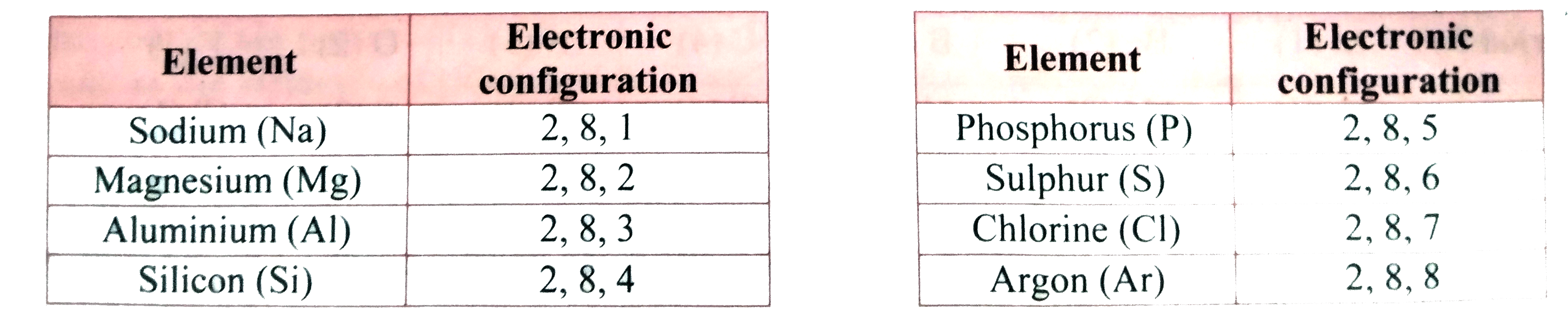
- The elements in the third period, namely, Na, Mg, AI, Si, P, S, CI and...
Text Solution
|
- What are the values of n for the shells L and M?
Text Solution
|
- What is the maximum number of electrons that can be accommodated in a ...
Text Solution
|
- Deduce the maximum electron holding capacity of the shells K, L and M?
Text Solution
|
- What is the relationship between the electronic configuration of an el...
Text Solution
|
- The atomic number of beryllium is 4 while that of oxygen is 8. Write d...
Text Solution
|
- The table on Textbook page no. 25 is made on the basis of the modern p...
Text Solution
|
- What is the periodic trend in the variation of valency while going fro...
Text Solution
|
- What is the periodic trend in the variation of valency while going dow...
Text Solution
|
- By referring to the modern periodic table, find out the periods to whi...
Text Solution
|
- State the period to which the above elements belong.
Text Solution
|
- Arrange the above elements in a decreasing order of their atomic radii...
Text Solution
|
- Does this arrangement match with the pattern of the second period of t...
Text Solution
|
- Why this arrangement of elements is similar to the above period of the...
Text Solution
|
- Which of the above elements have the biggest and the smallest atom ?
Text Solution
|
- By referring to the modern periodic table, find out the group to which...
Text Solution
|
- Arrange the above elements vertically downwards in an increasing order...
Text Solution
|
- Does this arrangement match with the pattern of the group 1 of the mod...
Text Solution
|