A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMODYNAMICS
PRADEEP|Exercise Interger Type questions|11 VideosTHERMODYNAMICS
PRADEEP|Exercise Assertion- Reason Type Questions|19 VideosTHERMODYNAMICS
PRADEEP|Exercise Multiple choice questions (NCERT)|10 VideosSYSTEMS OF PARTICLES AND ROTATIONAL MOTION
PRADEEP|Exercise Assertion- Reason Type questions|20 VideosWORK, ENERGY AND POWER
PRADEEP|Exercise Assertion-Reason Type Questions|24 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-THERMODYNAMICS-Multiple choice questions.
- Helium gas goes through a cycle ABCDA (consisting of two isochoric and...
Text Solution
|
- One mole of an ideal gas goes from an initial state A to final state B...
Text Solution
|
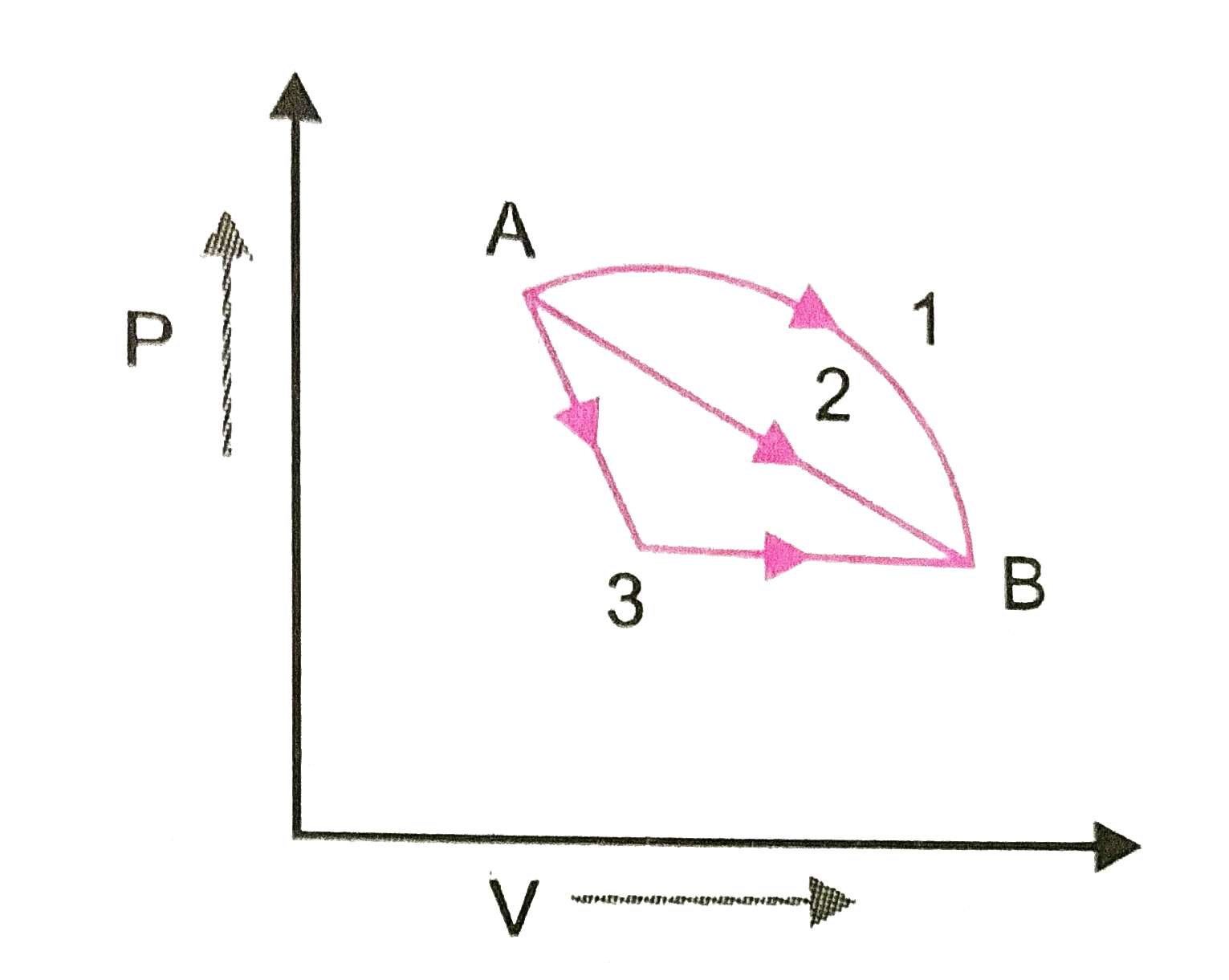
- An ideal gas goes from State A to state B via three different process ...
Text Solution
|
- During an adiabatic process, the pressure of a gas is found to be prop...
Text Solution
|
- The amount of heat energy required to raise the temperature of 1 g of ...
Text Solution
|
- A gas is taken through the cycle A rarrB rarr C rarr A, as shown in fi...
Text Solution
|
- Steam at 100^(@)C is passed into 20 g of water at 10^(@)C when water a...
Text Solution
|
- A body at a temperature of 727^(@)C and having surface area 5 cm^(2), ...
Text Solution
|
- A black body emit heat at the rate of 20 W, when its tempertaure is 22...
Text Solution
|
- According to Wien's law
Text Solution
|
- On observing light from three different stars P, Q and R, it was found...
Text Solution
|
- In (figure). shows two path that may be taken by a gas to go from a st...
Text Solution
|
- The two ends of a metal rod are maintained at temperature 100^(@)C and...
Text Solution
|
- An ideal gas is compressed to half its initial volume by means of seve...
Text Solution
|
- The value of coefficient of volume expansion of glycerin is 5 xx 10^(-...
Text Solution
|
- The balck body specturm of an object O(1) is such that its radiant int...
Text Solution
|
- Three rods of Copper, Brass and Steel are welded together to from a Y ...
Text Solution
|
- A solid body of constant heat capacity 1J//^@C is being heated by keep...
Text Solution
|
- A gas is compressed isothermally to half its initial volume. The same ...
Text Solution
|
- n' moles of an ideal gas undergoes a process AtoB as shown in the figu...
Text Solution
|