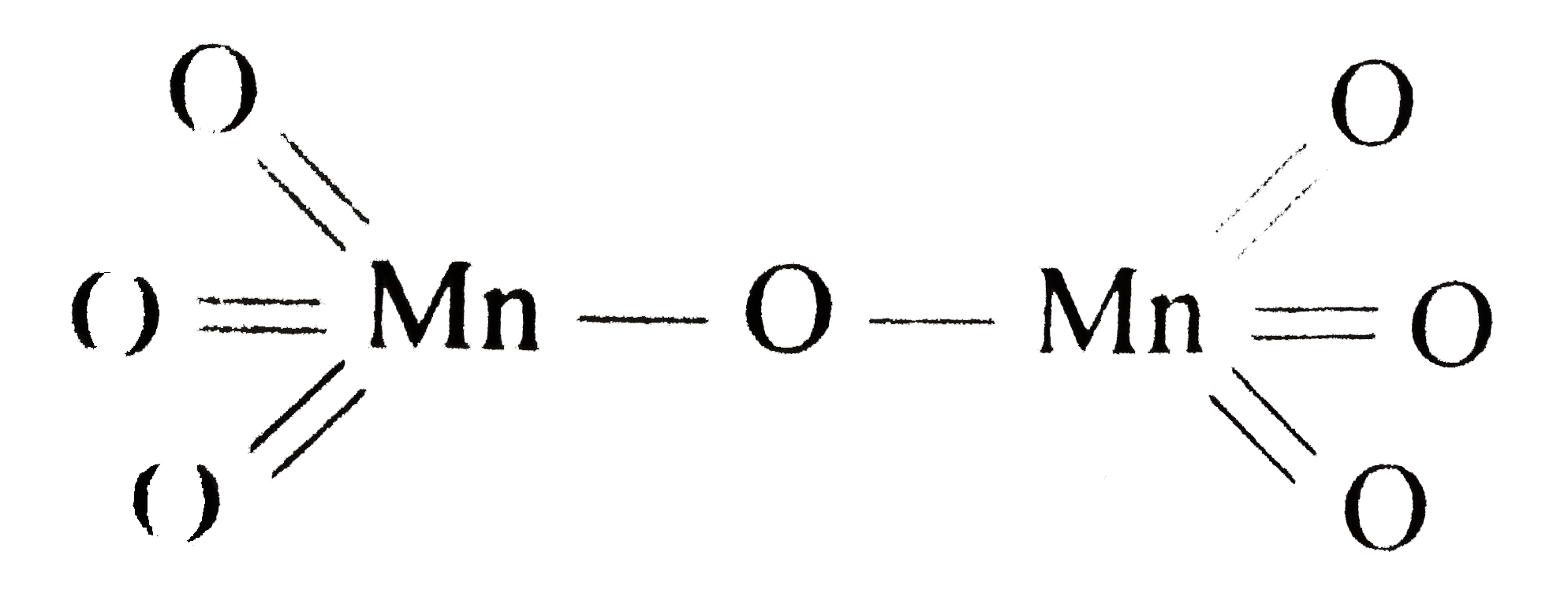A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
A2Z-THE D AND F BLOCK ELEMENTS-Section B - Assertion Reasoning
- Assertion : The number of unpaired electrons in the following gaseous ...
Text Solution
|
- Assertion : Lanthanoids show a limited of ixidation states wheres acta...
Text Solution
|
- Assertion : The highest manganese fluroide is MnF(4) and the highest o...
Text Solution
|
- Assertion: Mercury is not considered as a transition element. Reason...
Text Solution
|
- Assertion: In any transition series the magnetic moment of M^(2+) ions...
Text Solution
|
- Assertion : Silver intrate is reduced to silver by the hydrides of 15t...
Text Solution
|
- Assertion : Ag(2) S + 4 KCN 2K [Ag(CN)(2)] + K(2) s Reason : The rea...
Text Solution
|
- Assertion: K2Cr2O7 is used as primary standard in volumetric analysis....
Text Solution
|
- Assertion : The value of enthaply of atomisation is maximum at about t...
Text Solution
|
- Assertion: The spin only magnetic moment of Sc^(3+) is 1.73 BM. Reas...
Text Solution
|
- Assertion : Hydrochloric acid is not used to acidify a KMnO(4) solutio...
Text Solution
|
- Assertion : Solution of Na(2) CrO(4) in water is intensely electrons. ...
Text Solution
|
- Assertion : Reaction of thionyl chloride with hydrated ferric chloride...
Text Solution
|
- Assertion : Hyydroquinone is used as a developer for developing black ...
Text Solution
|
- Assertion : The order of atomic radii of Cu, Ag and Au is Cu lt Ag ~~ ...
Text Solution
|
- Assertion : 4d and 5d series elements have nearly same atomic radius. ...
Text Solution
|
- Assertion: Tungsten has very high melting point. Reason: Tungsten is...
Text Solution
|
- Assertion: Mn atom loses ns electrons first during ionisation as compa...
Text Solution
|
- Assertion : CuSO(4).5H(2)O on heating to 250^(@)C losses all the five ...
Text Solution
|
- Assertion : Silver chloride dissolves in execss ammonia. Reason : A...
Text Solution
|