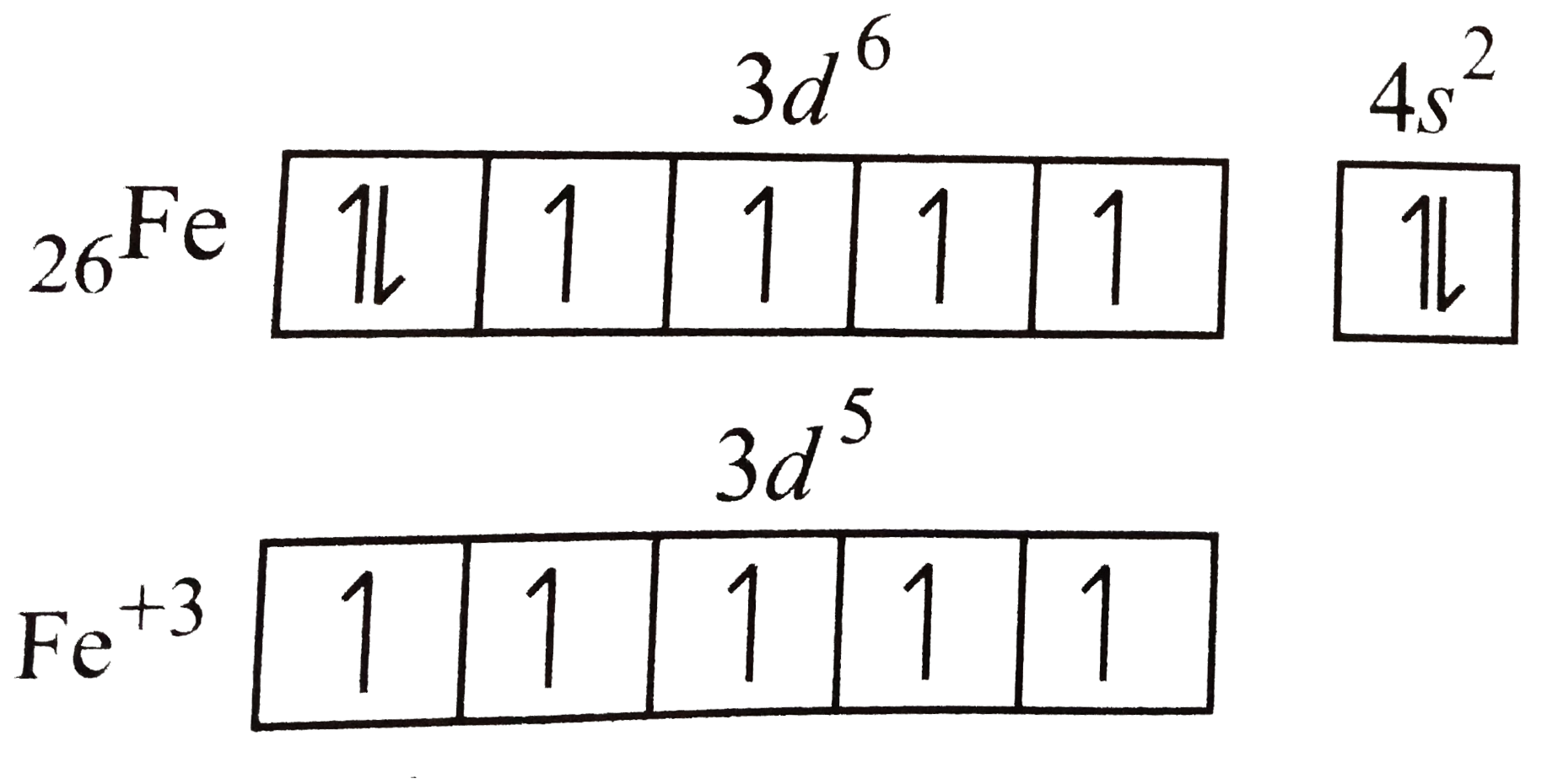A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
A2Z-THE D AND F BLOCK ELEMENTS-AIIMS Questions
- Mond's process is used for
Text Solution
|
- Stainless steel is an alloy of
Text Solution
|
- Percentage of silver in German silver is
Text Solution
|
- Brass in an ally of
Text Solution
|
- Most stable oxidation state of iron is
Text Solution
|
- F(2) is formed by reacting K(2) MnF(6) with
Text Solution
|
- Copper sulphate solution reacts with KCN to give (a) Cu(CN)(2) (b)...
Text Solution
|
- If excess of NH(4)OH is added to CuSO(4) solution, it forms blue colou...
Text Solution
|
- When metallic copper comes in contact with mositure, a green powdery/p...
Text Solution
|
- Which of the following does not react with AgCl?
Text Solution
|
- For making Ag from AgNO(3), Which of the following is used?
Text Solution
|
- ZnO when heated with BaO at 1100^(@)C gives a compound. Identify the c...
Text Solution
|
- Which of the following metals is obtained by leaching out process usin...
Text Solution
|
- Bessemer converter is used for Atomic nos, Mn = 25, Fe = 26, Co = 27...
Text Solution
|
- Conectrated aqueous sodium hydroxide can be a separated mixture of
Text Solution
|
- Which of the following oxoacide of phosphorus is a reducing agent and ...
Text Solution
|
- Among the following the compound that is both paramagnetic and coloure...
Text Solution
|
- What is IUPAC name of the following ?
Text Solution
|
- Which is least stable in aqueous medium
Text Solution
|
- Which of the following can be reduce easily
Text Solution
|