A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
A2Z-COORDINATION COMPOUNDS-Section D - Chapter End Test
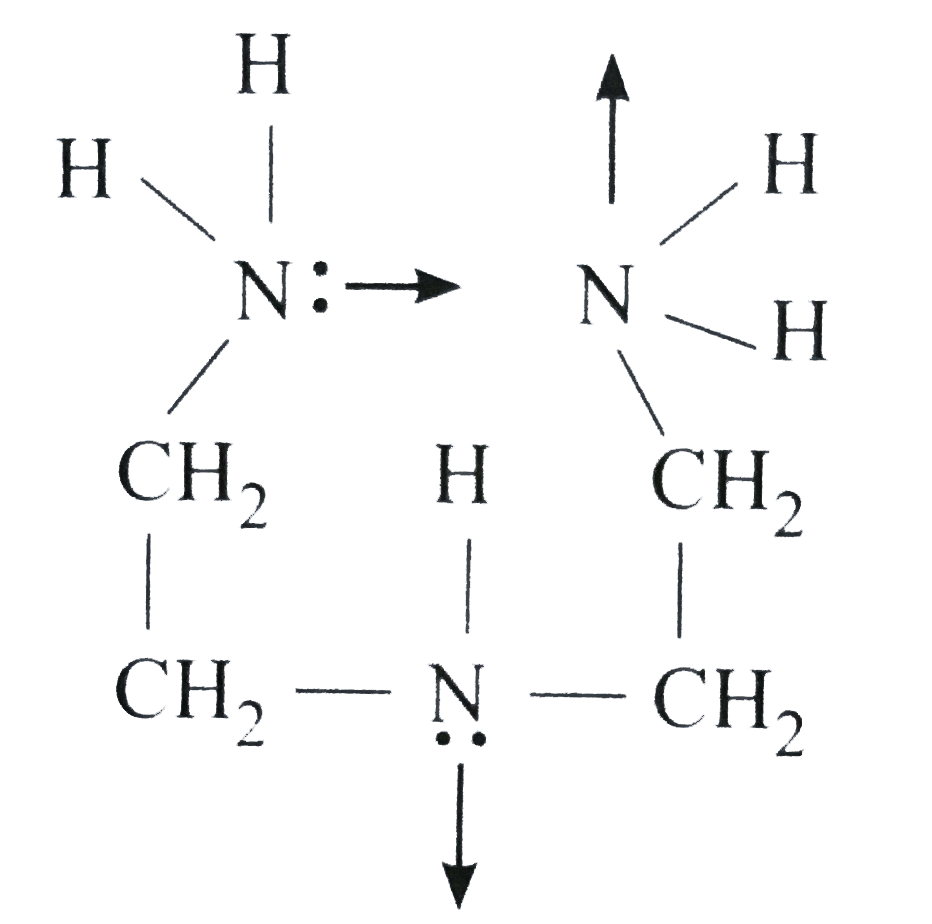
- Diethylene triamine is:
Text Solution
|
- Co-ordination number of Fe in the complexes [Fe(CN)6]^(4-), [Fe(CN)6]^...
Text Solution
|
- (Me)(2) SiCl(2) on hydrolysis will produce.
Text Solution
|
- Types of isomerism shown by [Cr(NH3)5NO2]Cl2 is
Text Solution
|
- Which of the following will no give a precipate with AgNO3?
Text Solution
|
- In the process of extraction of gold, Roasted gold ore +CN^(-)+H2Oov...
Text Solution
|
- A reagent used for identifying nickel ion is:
Text Solution
|
- Which of the following complex is an outer orbital complex?
Text Solution
|
- Which of the following has the largest number of isomers? .
Text Solution
|
- Which kind of isomerism is exhibited by octahedral [Co(NH(3))(4)Br(2)]...
Text Solution
|
- Which one of the following is expected to exhibit optical isomerism (e...
Text Solution
|
- The pair of the compounds in which both the metals are in the highest ...
Text Solution
|
- IUPAC name of [Co(NH3)5NO2]Cl2
Text Solution
|
- Which of the following is considered to be an anticancer species ?
Text Solution
|
- An aqueous solution of COCL2 on addition of excess of concentrated HCl...
Text Solution
|
- The correct order for the wavelength of absorption in the visible regi...
Text Solution
|
- In which of the following pairs both the complex show optical isomeris...
Text Solution
|
- Which of the following compounds shows optical isomerism?
Text Solution
|
- CN^(-) is a strong field ligand. This is due to the fact that
Text Solution
|
- Considering H2O as a weak field ligand, the number of unpaired electro...
Text Solution
|
- Given the molecular formula of the hexacoordinated complexes (A) [CoCl...
Text Solution
|