A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ORGANIC COMPOUNDS CONTAINING HALOGENS
A2Z|Exercise Elimination Reaction And Polyhalogen Compounds|67 VideosORGANIC COMPOUNDS CONTAINING HALOGENS
A2Z|Exercise Methods Of Preparationof Haloarenes|19 VideosORGANIC COMPOUNDS CONTAINING HALOGENS
A2Z|Exercise Section D - Chapter End Test|30 VideosMOCK TEST
A2Z|Exercise Mock Test 2|45 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-ORGANIC COMPOUNDS CONTAINING HALOGENS-Physical Properties And Nuleophilic Substitution Reaction
- For CH(3)Br+OHrarrCH(3)OH+Br the rate of reaction is given by the ex...
Text Solution
|
- What would be the produt when neopentyl chloride reacts with sodium et...
Text Solution
|
- The rate law for the reaction RCl + NaOH(aq) rarr ROH + NaCl is give...
Text Solution
|
- S(N)1 reaction is favoured by
Text Solution
|
- When ethyl bromide is treated with moist Ag(2)O main product is//are.
Text Solution
|
- Treatment of ammonia with excess of ethyl chloride will yield
Text Solution
|
- The reactivity of ethyl chloride is
Text Solution
|
- The reactivities of methy chloride propyl chloride and chlorobenzene a...
Text Solution
|
- Reaction of t-butyl bromide with sodium methoxide produces
Text Solution
|
- C(6)H(5)-CH(2)-CI+KCN(aq)rarrC(6)H(5)-CH(2)-C=N+KCI Compounds X and ...
Text Solution
|
- Ethylidence chloride on treatment with aqueous KOH gives .
Text Solution
|
- C(2)H(5)CI+KCNrarrX overset("Hydrolysis") rarr Y. X' and Y are
Text Solution
|
- The set of compounds in which the reactivity of halogen atom in the as...
Text Solution
|
- Viny chloride reacts with HCI to from
Text Solution
|
- When ethyl iodide is heated with silver nitrate the product obtained i...
Text Solution
|
- For a given alkyl group the densities of the halides follow the order
Text Solution
|
- Identify the major product
Text Solution
|
- HEATING ALKYL HALIDES WITH DRY SILVER OXIDE
Text Solution
|
- Alkyl halide can be converted into alkene by
Text Solution
|
- Among the following, the molecule with the highest dipole moment is :
Text Solution
|
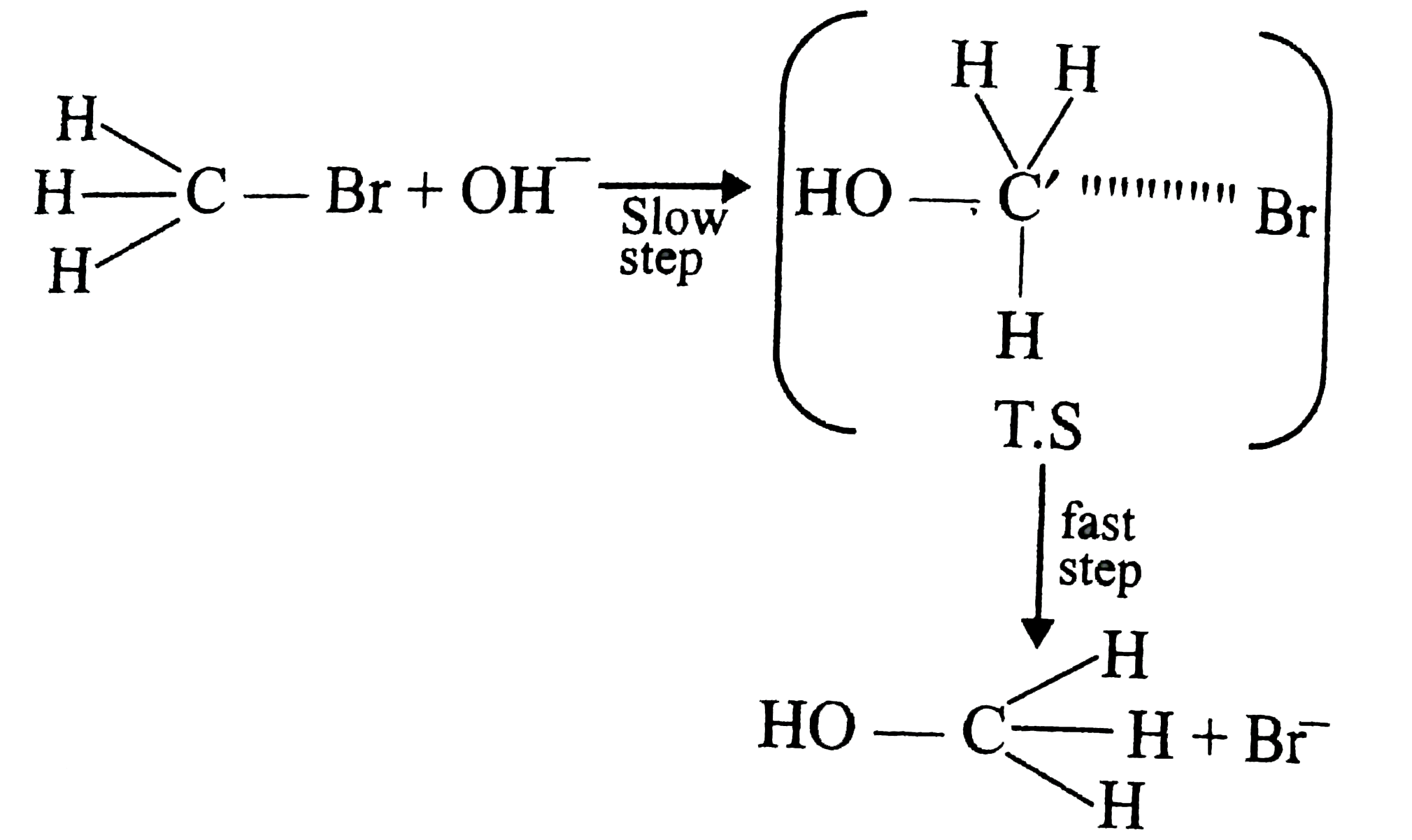 Rate of reaction` [r] =K [CH_(3) Br][OH]` .
Rate of reaction` [r] =K [CH_(3) Br][OH]` .