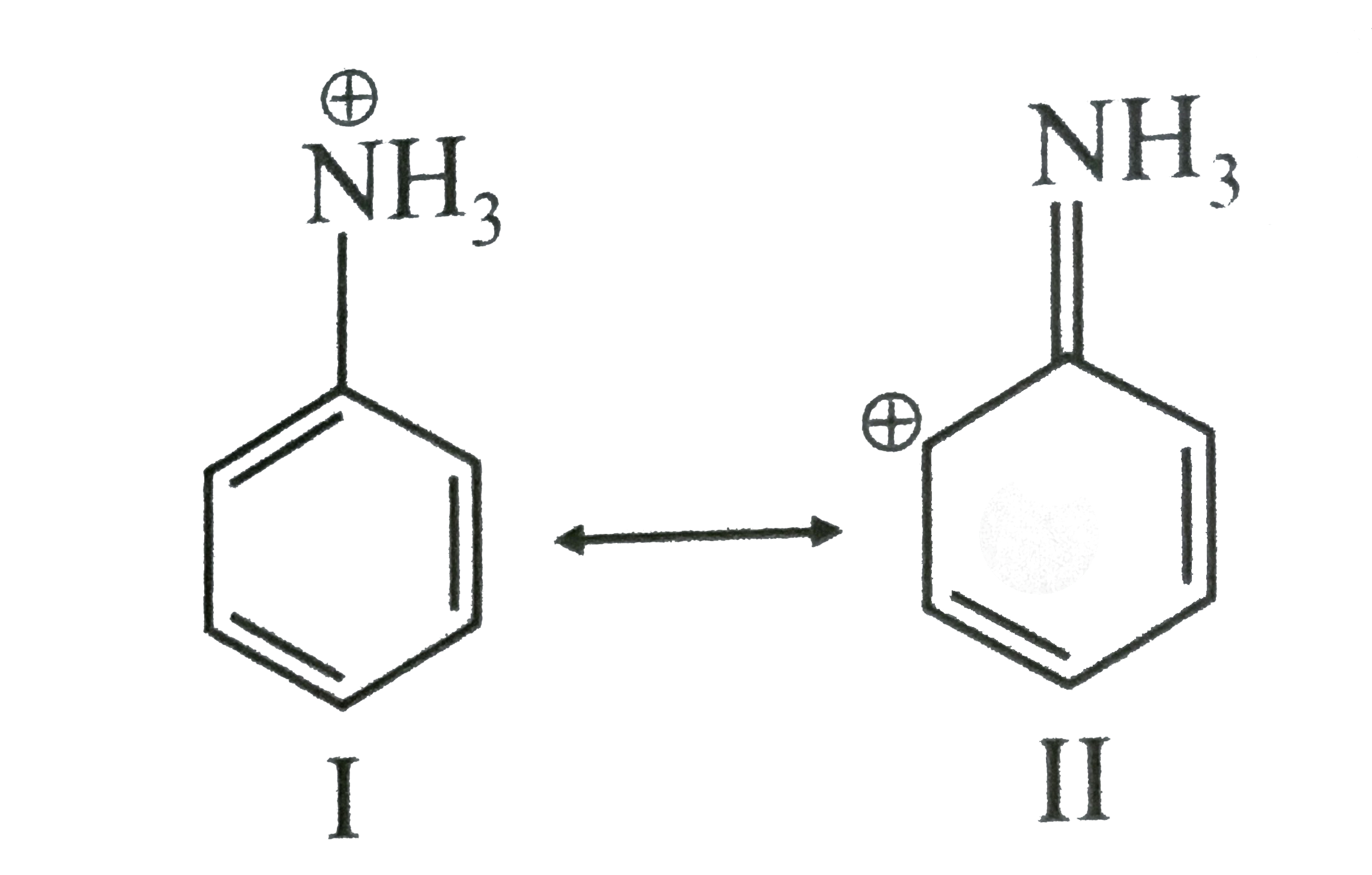
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ORGANIC COMPOUNDS CONTAINING NITROGEN
A2Z|Exercise Nitrocyanide And Isocyanides|32 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
A2Z|Exercise Section B - Assertion Reasoning|11 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
A2Z|Exercise Section D - Chapter End Test|30 VideosORGANIC COMPOUNDS CONTAINING HALOGENS
A2Z|Exercise Section D - Chapter End Test|30 VideosP BLOCK ELEMENTS ( GROUP 15,16,17,18)
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-ORGANIC COMPOUNDS CONTAINING NITROGEN-Aromatic Amines And Diazonium Salts
- Benzldehyde condenses with N, N-dimethylaniline in presence of anhydro...
Text Solution
|
- The final product C, obtained in this reaction
Text Solution
|
- In the chemical reactions the compounds (A) and (B) are .
Text Solution
|
- Boverset(H(2))underset(Ni)(rarr)Coverset(HNO(2))(rarr)D Aniline in ...
Text Solution
|
- In the chemical reaction, compounds A and B respectively are
Text Solution
|
- Azo dye is prepared by the coupling of phenol and:
Text Solution
|
- Among the following the strongest base is
Text Solution
|
- Amongst the compounds gives, the one that would form a brilliant color...
Text Solution
|
- C6H5NH2overset(NaNO2HCl)toXoverset(Cu2(CN)2)toYoverset((X2)/(H^(+)))to...
Text Solution
|
- Nitration of aniline also gives m-nitro anilime, in stron acidic mediu...
Text Solution
|
- Examine the following two structures for the anilinium ion and choose ...
Text Solution
|
- Which of the following is the strongest base:
Text Solution
|
- A positive carbylmine test is given by
Text Solution
|
- The colours of p-amino azobenzene is
Text Solution
|
- Aniline when diazotized in cold and then treated with dimethyl aniline...
Text Solution
|
Text Solution
|
- In the following reaction, the structure of the major product (X) is:
Text Solution
|
- Aniline reacts with acetaldehyde to form
Text Solution
|
- (A) overset(NaNO(2)//HCl)underset((ii)H(2)//Ni)underset(0-5^(@))(rarr)...
Text Solution
|
- In the reaction, the structure of the product T is:
Text Solution
|